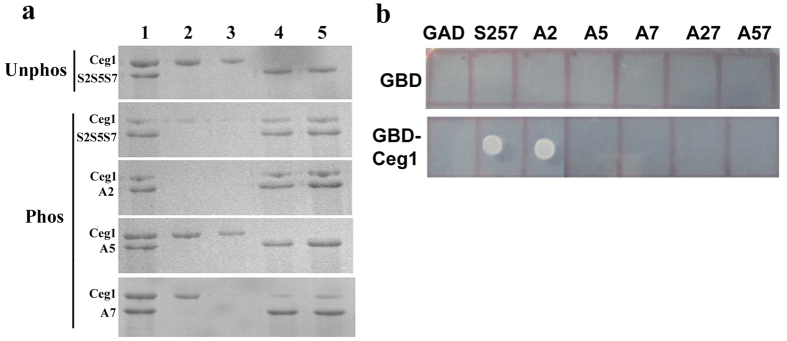
Figure 1. Ceg1 interacts with the phosphorylated Ser5 and Ser7 of CTD.
(a) SDS PAGE analysis of the pull down assay where Ceg1 does not bind to the unphosphorylated CTD, but binds to the CTD phosphorylated by Kin28. In the first panel, lane 1 represents the purified Ceg1 (5 μg) incubated with an equal amount of unphosphorylated CTD, lane 2 and 3 represents the washed samples and Lane 4 and 5 represents the samples eluted after the extensive wash of the complex. The subsequent panel (top to bottom) represents pull down complex for CTD phosphorylated at all the three serines (S2S5S7), S5S7, S2 and S2S5 respectively. (b) Yeast two hybrid assays, where GAD vector (pGADCU-1) alone or the GAD vector expressing either the consensus CTD with all the three Ser residues (Ser2Ser5Ser7 or S257) or Ser mutants (Ser2 mutant [A2] or Ser5 mutant [A5] or Ser7 mutant [A7] or Ser2 + Ser7 double mutant [A27] or Ser5 + Ser7 double mutant [A57]) was co-transformed into pJ69-4A strain either with GBD vector (pGBDCU-1) alone or GBD-Ceg1. The profiles show the growth due to the transcription of a HIS3 reporter, resulting from the interaction between two-hybrid fusion proteins.