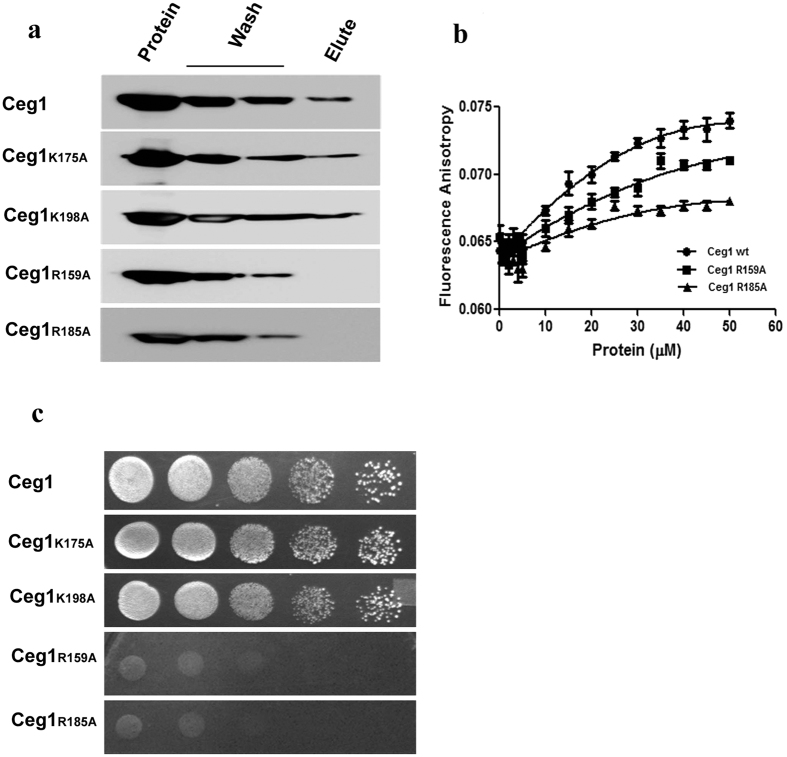
Figure 4. Arg159 and Arg185 of Ceg1 makes strong interaction with Ser5P of CTD.
(a) Immunodetection (anti-his) of the pull down sample where his-tagged Ceg1 or Ceg1 mutants (first lane) were incubated with CTD (14 repeats) phosphorylated by the Kin28 for their possible interaction. The samples were washed (two middle lanes) and subsequently the eluents were analyzed for the presence of Ceg1 or its mutants bound to the phosphorylated CTD (last lane). (b) The fluorescence anisotropy measurements, where 2 μM of FAM-CTD-Ser5P peptide was titrated against increasing concentrations of Ceg1 (•), Ceg1R159A (■) and Ceg1R185A (▲) respectively to find out their binding efficiency. (c) Yeast two hybrid assays, where GAD-CTD was co-transformed into pJ69-4A strain either with GBD-Ceg1 or with Ceg1 mutants (GBD-Ceg1K175A or GBD-Ceg1K198A or GBD-Ceg1R159A or GBD-Ceg1R185A). The profiles show the growth due to the transcription of a HIS3 reporter, resulting from the interaction between two-hybrid fusion proteins.