Figure 1.
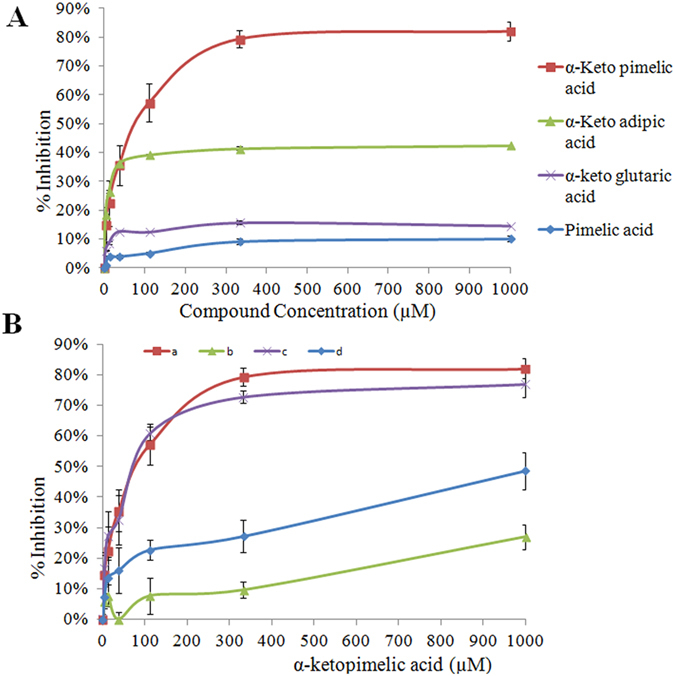
(A) IC50 analysis of pyruvate analogues: The percentage of inhibition by each inhibitor relative to control (in absence of inhibitor) was determined using the coupled assay. The protein was pre-incubated with each compound for 5 min at various concentrations (0–1000 uM) before the assay. Triplicate measurements were taken at each time point and data were plotted with mean ±1SD. (B) Competition analysis to infer the potential binding site of α-KPA: The competition experiments were conducted using the coupled assay after 5 min pre-incubation across various concentrations of α-KPA. Substrates concentrations were held constant at 500 μM of pyruvate and 400 μM of ASA in assay mix. The Mtb-rDapA protein was pre-incubated with (a) α-KPA (0-1 mM), (b) α-KPA + pyruvate (500 μM), (c) with α-KPA + ASA (400 μM), and (d) pyruvate (500 μM) before addition of other assay components. Each concentration point was measured at least in triplicate and data were plotted as mean ± 1SD.
