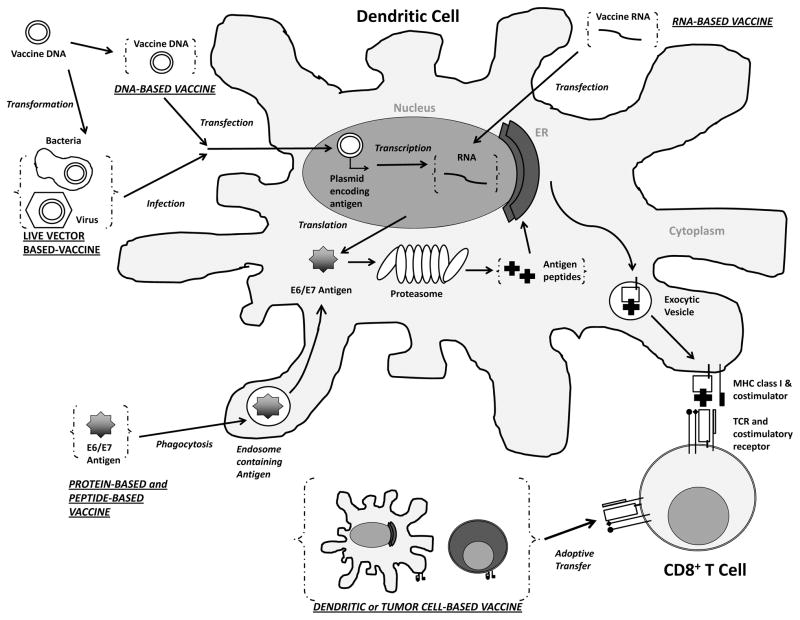
Figure 1. Immune activation by therapeutic HPV vaccination.
Administration of various types of therapeutic HPV vaccines leads to introduction of antigen into the body in different forms. DNA plasmids encoding antigens (HPV oncoprotein E6 and E7) can be transfected into dendritic cells through DNA vaccination or infection of modified live vector vaccine. The DNA encoding antigens will be transcribed into RNA, which can also be introduced into the cell through RNA vaccination. The transcribed RNA will be further translated into antigen proteins, or long peptides, which can also be taken up by the dendritic cells through phagocytosis following protein-based vaccination or peptide-based vaccination. The antigen proteins or long peptides are then processed by the proteasomes into short peptides, loaded onto class one major histocompatibility complex (MHC I) inside the endoplasmic reticulum (ER) to be presented to T cell receptors (TCR) of CD8+ T cells. Alternatively, dendritic cells or tumor cells can be prepared ex vivo to express target antigen on MHC I molecules as well as the necessary co-stimulatory molecules and be vaccinated back to the body (a process called adoptive transfer) as whole cell-based vaccines for the priming of T cells. (ER – Endoplasmic Reticulum; MHC – Major Histocompatibility Complex; TCR – T Cell Receptor; E6/E7 – Human Papillomavirus E6 and E7 Protein.)