Abstract
Objective
To identify early diagnostic profiles such as diagnostic codes and consultation patterns of cancer patients in primary care one year prior to cancer diagnosis.
Design
Total population-based case–control study.
Setting and subjects
4562 cancer patients and 17,979 controls matched by age, sex, and primary care unit. Data were collected from the Swedish Cancer Register and the Regional Healthcare Database.
Method
We identified cancer patients in the Västra Götaland Region of Sweden diagnosed in 2011 with prostate, breast, colorectal, lung, gynaecological, and skin cancers including malignant melanoma. We studied the symptoms and diagnoses identified by diagnostic codes during a diagnostic interval of 12 months before the cancer diagnosis.
Main outcome measures
Consultation frequency, symptom density by cancer type, prevalence and odds ratios (OR) for the diagnostic codes in the cancer population as a whole.
Results
The diagnostic codes with the highest OR were unspecified lump in breast, neoplasm of uncertain behaviour, and abnormal serum enzyme levels. The codes with the highest prevalence were hyperplasia of prostate, other skin changes and abdominal and pelvic pain. The frequency of diagnostic codes and consultations in primary care rose in tandem 50 days before diagnosis for breast and gynaecological cancer, 60 days for malignant melanoma and skin cancer, 80 days for prostate cancer and 100 days for colorectal and lung cancer.
Conclusion
Eighty-seven percent of patients with the most common cancers consulted a general practitioner (GP) a year before their diagnosis. An increase in consultation frequency and presentation of any symptom should raise the GP’s suspicion of cancer.
Key points
Knowledge about the prevalence of early symptoms and other clinical signs in cancer patients in primary care remains insufficient.
• Eighty-seven percent of the patients with the seven most common cancers consulted a general practitioner 12 months prior to cancer diagnosis.
• Both the frequency of consultation and the number of symptoms and diseases expressed in diagnostic codes rose in tandem 50–100 days before the cancer diagnosis.
• Unless it is caused by a previously known disease, an increased consultation rate for any symptom should result in a swift investigation or referral from primary care to confirm or exclude cancer.
Keywords: Cancer, consultation, diagnosis, general practice, ICD codes, primary healthcare, Sweden
Introduction
Today, despite advances in medical diagnostics, cancer is often detected at an advanced stage. One main reason is our limited understanding of the early symptoms that cancer patients present in primary care. There has been lack of consensus over whether delay in cancer diagnosis truly affects survival.[1–3] However, there is growing evidence that screening or early detection of symptomatic cancer results in a better prognosis for the patient.[4–9] Not only does this mean a higher chance of being cured, but also less need for toxic treatments, fewer side effects, better quality of life and health–economic-related benefits.
More than two-thirds of all cancers are diagnosed in primary care.[8,10–14] A general practitioner (GP) diagnoses a handful of the most common cancers during the year.[11,15,16] Patterns of increasing consultations before cancer diagnosis have been reported from primary care.[17,18] The professional challenge of GPs is to identify the relatively few cancer patients from among the many patients with symptoms that are mostly the same for benign diseases as well as for cancer. Cancer symptoms can present as alarm symptoms, such as a tumour or bleeding.[19] Cancer can also present with unspecific symptoms, and it is the GP’s task to determine if cancer is an underlying cause.[20–23] The knowledge about the prevalence of early symptoms and other clinical signs in cancer patients in primary care remains insufficient. We hypothesise that compared to non-cancer patients, cancer patients have different consultation (physical meeting) patterns and different diagnostic profiles one year prior to cancer diagnosis. Our primary aim is to identify, describe and assess early diagnostic profiles such as diagnostic codes and consultation patterns of cancer patients in primary care one year prior to their cancer diagnosis.
Material and methods
Study design
We designed a total population-based, case–control study using the Swedish Cancer Register and Regional Healthcare Database in Västra Götaland Region. This region, which has 1.6 million inhabitants (17% of the Swedish population), is situated in southwest Sweden and has rural and urban areas. Its demographic composition is representative of the whole of Sweden. Cancer patients and matched controls meeting the inclusion criteria (below) were investigated for primary care diagnostic profiles.
Databases
The Swedish Cancer Registry
The Swedish Cancer Registry, which was founded in 1958, is one of the oldest registries in Sweden and has high validity.[24] All physicians and pathologists in Sweden are obliged by law to report all incident cases of cancer in both living and dead patients to the registry. During 2011, 57,726 cases of cancer were reported to the registry.[25] Each patient has a unique personal identity number, which all Swedish residents acquire either at birth or on immigration to Sweden.
VEGA database
The administrative regional healthcare database (VEGA), which was established in 2000, covers all hospitals, specialised outpatient care, and all private and public primary healthcare centres. The database includes place of residence, age, sex, healthcare contacts, and diagnostic codes for diagnoses and surgical procedures.[26] Regular medical revisions have been made of this database for the diagnostic accuracy. At each consultation, physicians are obliged to enter codes for patients’ current diseases or symptoms into the patients’ medical records. The reimbursement system to primary care providers is partly based on the disease burden of the patients, which is identified by diagnostic codes reported to the regional healthcare databases.[26]
The study population
Identification of cases
Cases eligible for our study were identified from the Swedish Cancer Register for the period 1 January 2011 to 31 December 2011. Inclusion criteria were: (1) being diagnosed in Västra Götaland Region with prostate cancer, breast cancer, colorectal cancer, lung cancer, gynaecological cancer or skin cancers including malignant melanoma; (2) being alive at the time of the cancer diagnosis; (3) being age 18 years or older; and (4) having visited a GP in the year before the cancer diagnosis. Individuals were excluded from participation if they: (1) lacked controls or (2) had a previous cancer diagnosis in the Swedish Cancer Register 1991–2010.
Generation of controls
The controls were selected from the regional healthcare database. They had the same inclusion criteria as the cancer patients except for not being diagnosed with cancer. Only controls from the region that had visited a GP in primary care from 1 January 2010 to 31 December 2011 were eligible. Four controls were matched to each case using three criteria: age, sex and primary care unit.
Data collection and study measurements
The unique personal identity numbers of both cases and controls were linked to VEGA. We collected all data concerning diagnoses and dates of consultations with a GP from 1 January 2010 to 31 December 2011. The data extracted were assigned diagnostic codes according to the International Statistical Classification of Diseases and Related Health Problems – 10th Revision (ICD-10) Swedish version or the Classification of Diseases and Health Problems 1997 Primary Care (KSH97-P), which is an abbreviated version of ICD-10 adapted to Swedish primary care to facilitate diagnostic coding.[27–29] We arranged the codes according to their incidence in the study population and excluded diagnostic codes that occurred less frequently than in 1% of the study population. We reduced the number of diagnostic codes by merging ICD-10 four-character diagnostic codes and KSH97-P codes to the closest three-character diagnostic code. The three-character codes are also the “core” classifications and mandatory levels codes for reporting to the WHO mortality database and for general international comparisons.[30] A smaller number of diagnostic codes, mostly for symptoms, could not be merged into three characters ICD-10 codes, as their clinical significance would be lost as a result (e.g. the four-character diagnostic codes for K59.0 Constipation and K51.1 Functional diarrhoea were not merged into the three-character code K59 Other functional intestinal disorders).
Data analysis
The consolidated diagnostic groups were used as variables for univariable conditional logistic regression with the outcome being cancer Yes or No. This gave us a list of variables associated with each cancer type as well as their respective odds ratios (OR). The diagnostic codes with OR less than 1 were omitted.
Using consultation dates from the primary care data, we calculated the lead time between consultation and cancer diagnosis. We then plotted the consultation frequency over time, expressed as weekly consultation frequency of cancer patients compared to controls as well as symptom density, expressed as weekly diagnostic code frequency over time for cancer patients in the year prior to cancer diagnosis as well as for their controls. All analyses were done in the statistical software R version 3.0.1.
Data entry and management of data files
All data collected were kept safe and confidential. The electronic files were entered into computerised data files and stored on a password protected and encrypted hard drive. Each patient was assigned a study identity number to ensure confidentiality. The key for identification was kept separate. All patient identifiers were removed from the analytic datasets. Only the authorised project staff had access to the study database. All data will be safely stored for 10 years to enable revision.
Results
Cases and controls
From the 10,073 newly diagnosed cancers in Västra Götaland Region in 2011, we identified 6486 patients with any of the seven target tumour types, alive at diagnosis and older than 18 years. Of the 6486 patients 87% visited a GP the year before diagnosis. A total of 5669 patients met the inclusion criteria (Figure 1). Individuals were excluded from participation if they: (1) lacked controls or (2) had a previous cancer diagnosis in the Swedish Cancer Register 1991–2010. A total of 4562 cancer patients were included in the study. The median age of cancer patients at diagnosis was 68 years, and males and females were equally represented (Table 1).
Figure 1.
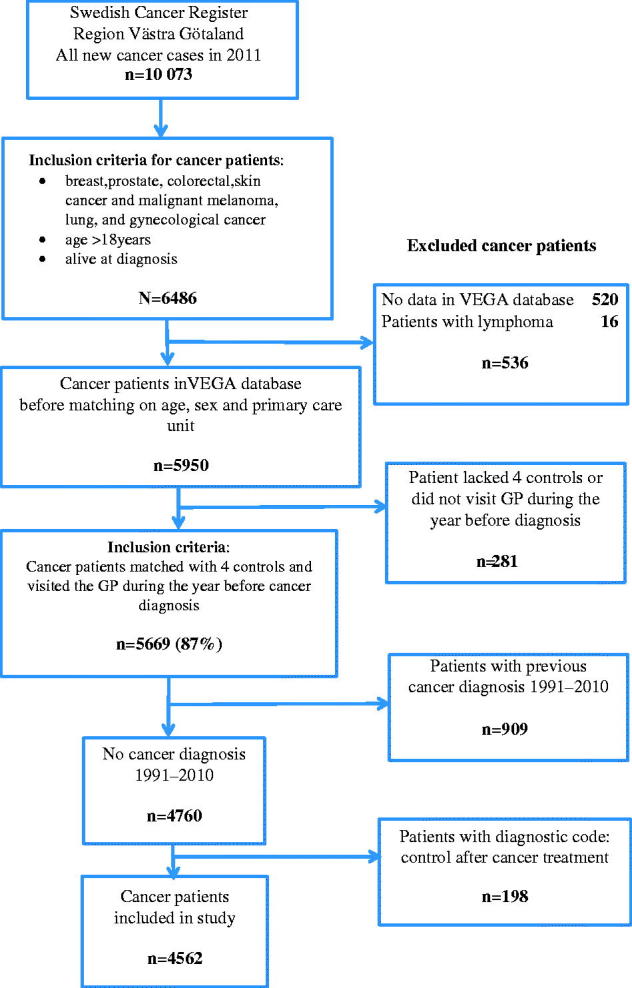
Identification of cancer patients included in the study.
Table 1.
Characteristics of the cancer patient population.
| Type of cancer | Total number, n (%) | Men, n (%) | Women, n (%) | Median age at diagnosis [range] years |
|---|---|---|---|---|
| Breast | 947 (21) | 6 (1) | 941 (99) | 65 [29–97] |
| Colorectal | 753 (16) | 380 (50) | 373 (50) | 71 [31–95] |
| Gynaecological | 327 (7) | n.a* | 327 | 67 [30–91] |
| Lung | 373 (8) | 195 (52) | 178 (48) | 70 [31–94] |
| Malignant melanoma | 459 (10) | 212 (46) | 247 (54) | 63 [28–97] |
| Prostate | 1257 (28) | 1257 | n.a* | 68 [44–96] |
| Skin | 446 (10) | 222 (50) | 224 (50) | 79 [33–98] |
| All cancers included | 4562 (100) | 2272 (50) | 2290 (50) | 68 [28–98] |
n.a = non-applicable.
In total, 18,248 matched controls were originally generated but 269 were excluded because they died before their cases were diagnosed with cancer and so, there were not always four controls for each case. Thus 17,979 controls were included in the study.
Diagnostic codes
We initially had more than 6000 different diagnostic codes and after merging them ended up with 575. These were used as variables for univariable conditional logistic regression. In the cancer population, the three most prevalent diagnostic codes with OR above 1 were hyperplasia of prostate, other skin changes and abdominal and pelvic pain (Table 2). However, the three diagnostic codes with highest OR were unspecified lump in breast, neoplasm of uncertain behaviour and abnormal serum enzyme levels (Table 2).
Table 2.
Most prevalent diagnostic codes in the cancer population, ICD-10 classification.
| Diagnostic codes | Prevalence | OR (95% CI)* |
|---|---|---|
| N40 Hyperplasia of prostate | 11.5 | 2.6 (2.3–3.0) |
| R23 Other skin changes | 10.4 | 2.2 (2.0–2.5) |
| R10 Abdominal and pelvic pain | 9.0 | 1.5 (1.3–1.7) |
| D48 Neoplasm of uncertain or unknown behaviour of other and unspecified sites | 5.8 | 6.5 (5.3–7.9) |
| D64 Other anaemias | 5.5 | 1.9 (1.7–2.3) |
| L57.0 Actinic keratosis | 4.9 | 1.9 (1.6–2.3) |
| R74 Abnormal serum enzyme levels | 4.2 | 5.7 (4.5–7.2) |
| N63 Unspecified lump in breast | 4.2 | 18.5 (13.1–26.0) |
OR = odds ratio calculated between cases and controls. Diagnostic codes with OR <1 are omitted. CI = confidence interval.
p Value <0.05.
Symptom frequency and symptom density
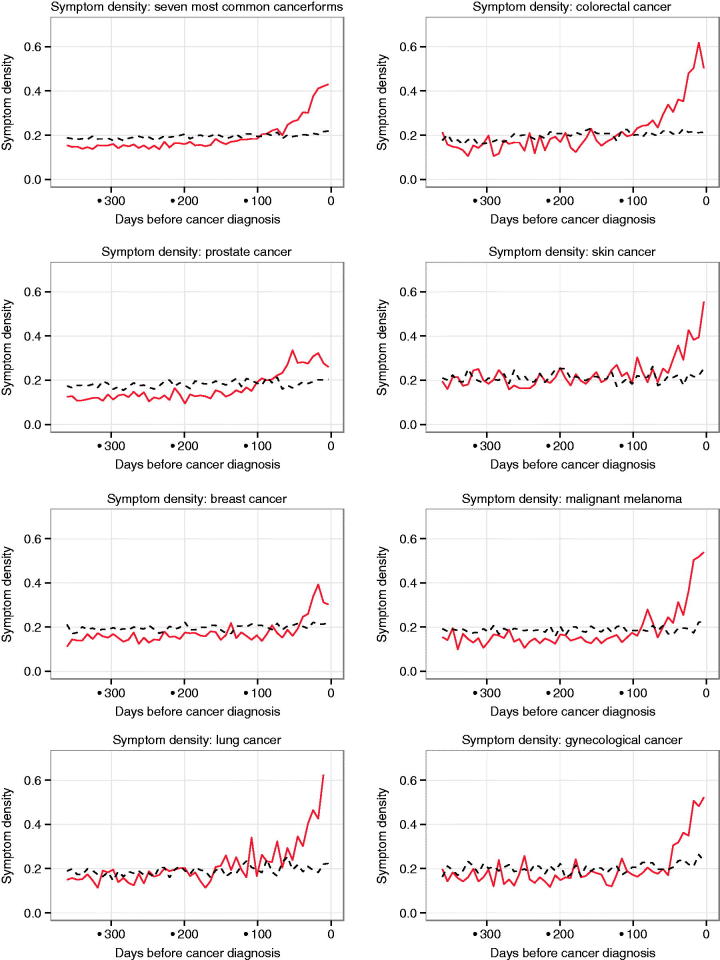
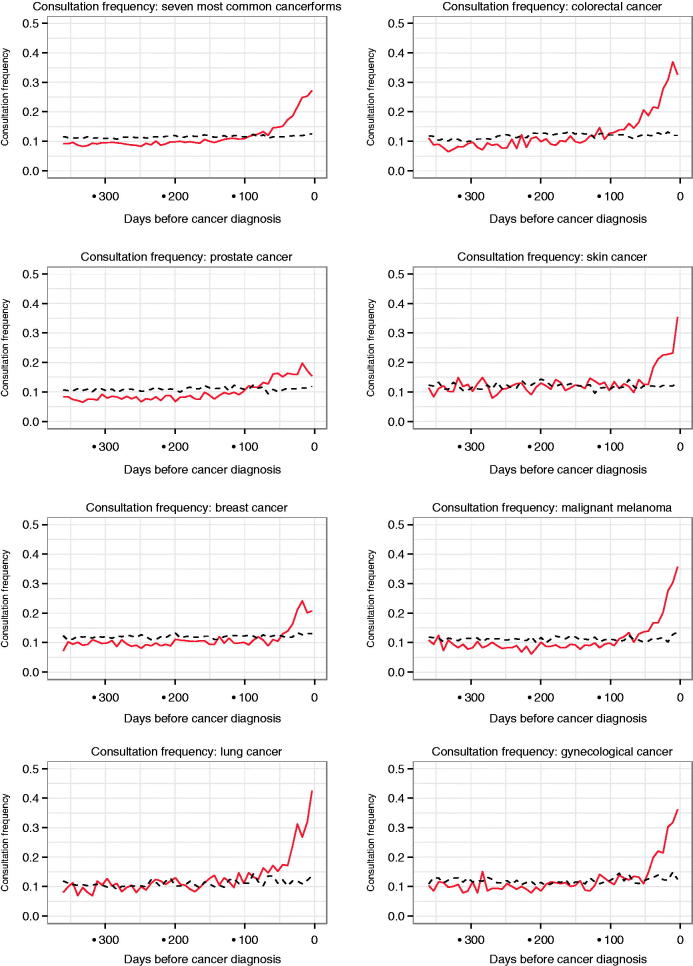
The frequency of diagnostic codes, which is presented in Figure 2 as symptom density, and the frequency of consultations start to rise approximately simultaneously before cancer diagnosis (Figure 3). Depending on cancer type, the pattern is different. Cancers that present with alarm symptoms, such as palpable or visual changes as breast cancer and malignant melanoma, have the shortest diagnostic interval of 50–60 days. Cancers such as prostate cancer and lung cancer that present with more vague, unspecific symptoms have a rising trend of consultation frequency in primary care and diagnostic code frequency starting 80–100 days prior to cancer diagnosis.
Figure 2.
Symptom density: weekly diagnostic code frequency of cancer patients (red continuous line) compared to controls (black interrupted line) one year prior to cancer diagnosis.
Figure 3.
Consultation frequency: weekly consultation frequency of cancer patients (red continuous line) compared to controls (black interrupted line) one year prior to cancer diagnosis.
Discussion
Summary
We found that 87% of the patients with the seven most common cancers consulted a GP 12 months prior to cancer diagnosis. Both consultation frequency and the number of symptoms and diseases expressed in diagnostic codes increased simultaneously 50–100 days before the cancer diagnosis. The diagnostics codes with highest OR were unspecified lump in breast, neoplasm of uncertain behaviour, and abnormal serum enzyme levels. However, the most prevalent diagnostic codes in the cancer population were hyperplasia of prostate, other skin changes and abdominal and pelvic pain.
Strength and weaknesses
The main strength of our study is that we included the total population of all adult patients with the seven most common cancers in a large region in Sweden, covering more than half of the annual cancer incidence. Another strength is that all our data are derived from reliable regional databases with an almost complete coverage of cancer diagnoses and diagnostic codes from primary care. All our diagnostic codes were registered before cancer diagnosis and automatically retrieved, thus avoiding selection bias. Another advantage is that it is a total population study in a region with 1.6 million inhabitants with a study population of nearly 23,000 patients.
Using diagnostic information directly from healthcare databases is both a strength and a weakness. Data collected from regional healthcare databases will not capture all symptoms or diseases that our study patients presented to their GPs. Using only coded information to answer research questions may miss vital information recorded in the text of the medical record. Studies that derive data directly from patients’ medical records usually present a higher frequency of recorded symptoms.[31] Since it is mandatory for the Swedish GPs and secondary care physicians to code due to the reimbursement system, an extensive and reliable amount of data is available. Some prevalent diagnostic codes in our study are most probably prevalent due to repeated registration in the medical record made by the GP from the previous consultation.
Prostate cancer was chosen as it is one of the most common cancers. We found an increased OR of hyperplasia of prostate among prostate cancer patients, but this finding could simply be a detection bias, as most prostate cancers today are diagnosed because of an elevated PSA.
Comparison with other studies
To our knowledge, no previous studies have examined the prevalence and OR of diagnostic codes of a representative cancer population as a whole, using the seven most common cancers in general practice. The studies that have presented OR of symptoms or clinical findings of different cancers looked at each cancer separately and therefore their results are not comparable with our study. For example, in our study unspecified lump in breast had an OR of 18.5 when studied as a part of a cancer population in primary care, while in a British study the OR was 110 for breast lump associated with breast cancer.[32]
A national population-based study of all incident cancers in Denmark diagnosed between 2001 and 2006 showed an increase in GP consultation patterns 5–6 months before diagnosis.[33] That differs from our study, which shows a rise in both the frequency of diagnostic codes and consultations in primary care for the most common cancers no more than 50–100 days prior to cancer diagnosis. This difference could be explained by the GPs in Sweden taking action in the diagnostic process more swiftly when their patients present with symptoms in which cancer can be a differential diagnosis. Another explanation could be that the Swedish GPs have a quicker access to diagnostic modalities. A study from the UK estimated a mean symptom lead time (the time from the presentation of symptoms caused by cancer in primary care and the diagnosis of cancer) of between 4.1 and 6.0 months, and medians between 2.0 and 3.2 months for lung and colorectal cancer.[34] This is comparable with the timings in our study, but we were not restricted in only studying symptoms caused by cancer but all diagnostic codes reported in the study population.
In our study, we examined the diagnostic interval in terms of days before cancer diagnosis but our findings are in consistency with other studies showing variation in the number of times patients visit their GP before hospital referral for suspected cancer depending on cancer type. Findings from the 2010 National Cancer Patient Experience Survey in England showed that patients with breast cancer and malignant melanoma had fewer numbers of visits compared to patients with lung cancer.[13]
Due to concerns about the diagnostic interval, countries like Denmark, Norway, and the United Kingdom have implemented urgent referral cancer pathways. Sweden has been inspired by them and also started to implement standardised cancer pathways during 2015. Even if that results in shorter diagnostic intervals and, hopefully, a better prognosis for the patients, studies in Denmark where Cancer Patient Pathways have been implemented since 2008, show that less than 40% of cancer patients were identified through those pathways.[35]
Conclusions
The vast majority of patients with the most common cancers do consult a GP a year before their diagnosis and therefore could be identified by the GP. When patients in primary care increase their consultation frequency and presentation of any symptom, it should raise the GP’s suspicion of a common cancer and result in a swift diagnostic activity to confirm or exclude cancer. Even with implementation of urgent referral pathways for different cancers, the vigilance of the GP regarding patients’ symptom presentation and consultation frequency will be as important as ever.
Acknowledgements
The authors thank Erik Holmberg PhD, statistician at Regional Cancer Center West for the extraction of data from the Swedish Cancer Register. We also thank Kristina Narbro, PhD, and Mona-Lis Dalbrekt, Department of Health Care Evaluation, County Council, Västra Götaland Region, for help in the extraction of data from VEGA.
Disclosure statement
The authors declare that they have no competing interests.
Funding information
The study was conducted without external funding. Access to the regional healthcare database VEGA was financed by Regional Cancer Center West, Sahlgrenska University Hospital, Gothenburg, Sweden.
Ethics
The Regional Ethical Review Board in Gothenburg has approved the study protocol (252-12).
References
- 1.Neal RD. Do diagnostic delays in cancer matter? Br J Cancer. 2009;101:S9–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rupassara KS, Ponnusamy S, Withanage N, et al. A paradox explained? Patients with delayed diagnosis of symptomatic colorectal cancer have good prognosis. Colorectal Dis. 2006;8:423–429. [DOI] [PubMed] [Google Scholar]
- 3.Iversen LH, Antonsen S, Laurberg S, et al. Therapeutic delay reduces survival of rectal cancer but not of colonic cancer. Br J Surg. 2009;96:1183–1189. [DOI] [PubMed] [Google Scholar]
- 4.Jones R, Latinovic R, Charlton J, et al. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. 2007;334:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabar L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260:658–663. [DOI] [PubMed] [Google Scholar]
- 6.Lindholm E, Brevinge H, Haglind E.. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008;95:1029–1036. [DOI] [PubMed] [Google Scholar]
- 7.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. [DOI] [PubMed] [Google Scholar]
- 8.Hansen RP. Delay in the diagnosis of cancer [PhD thesis]. Aarhus, Denmark: University of Aarhus; 2008. [Google Scholar]
- 9.Torring ML, Frydenberg M, Hansen RP, et al. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. 2013;49:2187–2198. [DOI] [PubMed] [Google Scholar]
- 10.Allgar VL, Neal RD.. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients. Br J Cancer. 2005;92:1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Månsson J. The diagnostic process of cancer from the general practitioner’s point of view [PhD thesis]. Göteborg: Göteborgs Universitet; 1999. [Google Scholar]
- 12.Vedsted P, Hansen RP, Bro F.. [General practice and early cancer diagnosis]. Ugeskr Laeger. 2011;173:1712–1715. [PubMed] [Google Scholar]
- 13.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13:353–365. [DOI] [PubMed] [Google Scholar]
- 14.Demagny L, Holtedahl K, Bachimont J, et al. General practitioners’ role in cancer care: a French-Norwegian study. BMC Res Notes. 2009;2:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101:S80–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingebrigtsen SG, Scheel BI, Hart B, et al. Frequency of ‘warning signs of cancer’ in Norwegian general practice, with prospective recording of subsequent cancer. Fam Pract. 2013;30:153–160. [DOI] [PubMed] [Google Scholar]
- 17.Dommett RM, Redaniel MT, Stevens MCG, et al. Features of childhood cancer in primary care: a population-based nested case-control study. Br J Cancer. 2012;106:982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton W, Round A, Sharp D, et al. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svendsen RP, Støvring H, Hansen BL, et al. Prevalence of cancer alarm symptoms: a population-based cross-sectional study. Scand J Prim Health Care. 2010;28:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen TN, Hansen RP, Vedsted P.. [Symptom presentation in cancer patients in general practice]. Ugeskr Laeger. 2010;172:2827–2831. [PubMed] [Google Scholar]
- 21.Hamilton W. Cancer diagnosis in primary care. Br J Gen Pract. 2010;60:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtedahl KA. The value of warning signals of cancer in general practice. Scand J Prim Health Care. 1987;5:140–143. [DOI] [PubMed] [Google Scholar]
- 23.Scheel BI, Holtedahl K.. Symptoms, signs, and tests: the general practitioner’s comprehensive approach towards a cancer diagnosis. Scand J Prim Health Care. 2015;33:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48:27–33. [DOI] [PubMed] [Google Scholar]
- 25.Socialstyrelsen Cancer incidence in Sweden 2011. 2012. Available from: http://www.socialstyrelsen.se/publikationer2012/2012-12-19. [Google Scholar]
- 26.Bjorck S, Palaszewski B, Friberg L, et al. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. 2013;44:3103–3108. [DOI] [PubMed] [Google Scholar]
- 27.Socialstyrelsen Internationell statistisk klassifikation av sjukdomar och relaterade hälsoproblem-systematisk förteckning (ICD-10-SE); 2010. [Google Scholar]
- 28.Socialstyrelsen Klassifikation av sjukdomar och hälsoproblem 1997. Primärvård: Socialstyrelsen; 1997. [Google Scholar]
- 29.Nystrom M, Vikstrom A, Nilsson GH, et al. Enriching a primary health care version of ICD-10 using SNOMED CT mapping. J Biomed Semantics. 2010;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organisation International Statistical Classification of Diseases and Related Health Problems – 10th Revision, Volume 2, Instruction Manual. Geneva: WHO; 2010. [Google Scholar]
- 31.Ford E, Nicholson A, Koeling R, et al. Optimising the use of electronic health records to estimate the incidence of rheumatoid arthritis in primary care: what information is hidden in free text? BMC Med Res Methodol. 2013;13:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker S, Hyde C, Hamilton W.. Risk of breast cancer in symptomatic women in primary care: a case-control study using electronic records. Br J Gen Pract. 2014;64:e788–e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen K, Fenger-Gron M, Flarup K, et al. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis – a population-based nationwide registry study of 127,000 incident adult cancer patients. BMC Health Serv Res. 2012;12:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas M, Ades AE, Hamilton W.. Symptom lead times in lung and colorectal cancers: what are the benefits of symptom-based approaches to early diagnosis? Br J Cancer. 2015;112:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen H, Torring M, Olesen F, et al. Cancer suspicion in general practice, urgent referral and time to diagnosis: a population-based GP survey and registry study. BMC Cancer. 2014;14:636. [DOI] [PMC free article] [PubMed] [Google Scholar]