Abstract
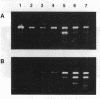
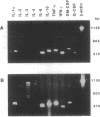
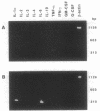
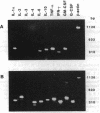
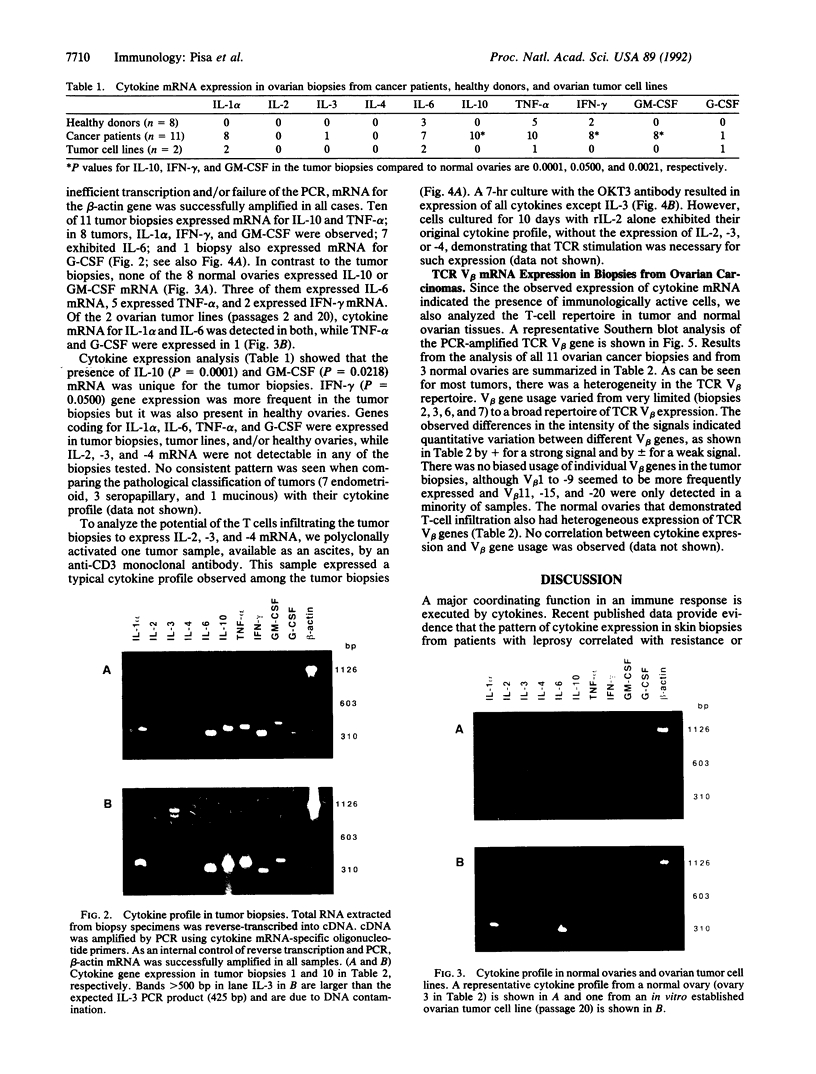
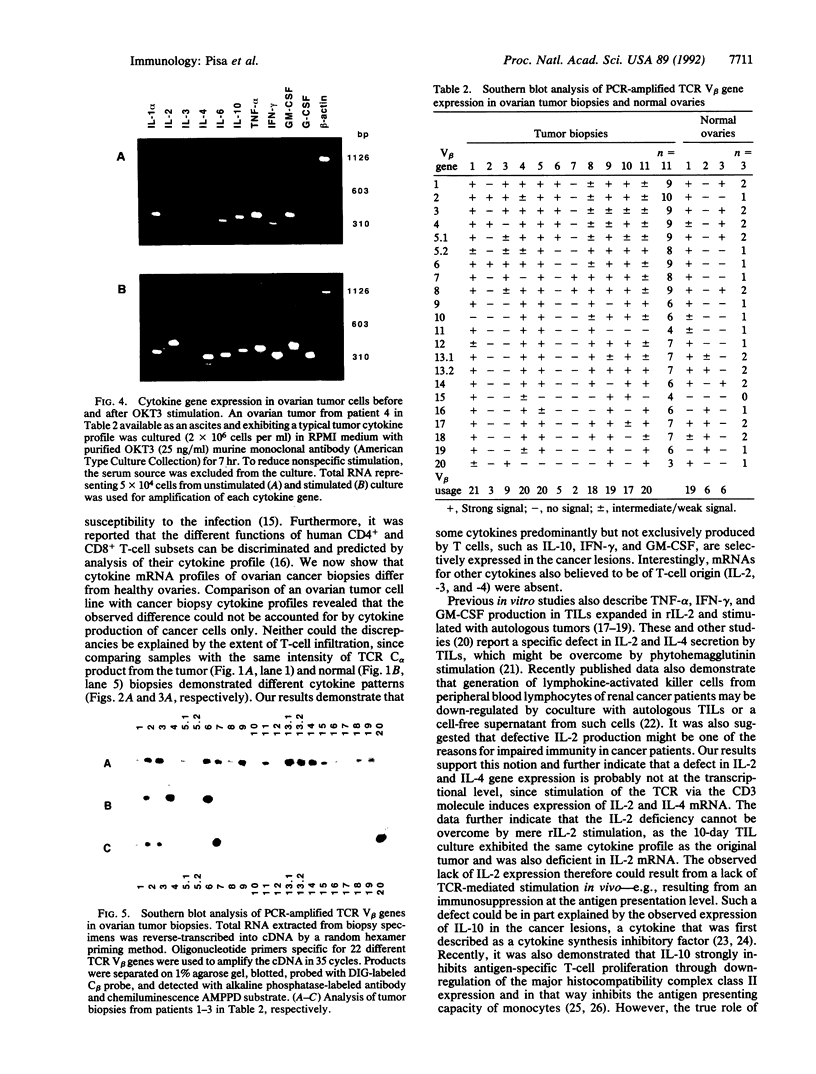
The variable clinical response seen with most cancer immunotherapy suggests that there is a large interindividual variation in immunologic response to tumors. One of the key functional parameters of an immune response is the local production of cytokines. As a method to survey the immune status of tumor-infiltrating cells, we have investigated the constitutive expression of cytokine mRNA in biopsies from epithelial ovarian carcinomas by using a PCR-assisted mRNA amplification assay. Using a set of cytokine-specific primers for 10 different cytokines, we have found selective expression of interleukin 10 (IL-10), granulocyte-macrophage colony-stimulating factor, and interferon gamma mRNA in ovarian tumor tissue as compared to normal ovaries and ovarian tumor cell lines. Such differences could not be explained by the extent of T-cell infiltration, since comparing samples with the same intensity of T-cell receptor (TCR) constant region alpha-chain product from the tumor and normal biopsies demonstrated different cytokine patterns. No IL-2 gene expression was detected in the tumor biopsies. IL-2 mRNA, however, became expressed after stimulation of the tumor-derived cells via the CD3 molecule but not after growth in recombinant IL-2 alone. Using the same methodology, we also analyzed the TCR variable region beta-chain gene repertoire. No restriction or biased expression of these genes was observed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y. The potential relevance of cytokines to ovarian physiology: the emerging role of resident ovarian cells of the white blood cell series. Endocr Rev. 1990 Aug;11(3):454–464. doi: 10.1210/edrv-11-3-454. [DOI] [PubMed] [Google Scholar]
- Belldegrun A., Kasid A., Uppenkamp M., Topalian S. L., Rosenberg S. A. Human tumor infiltrating lymphocytes. Analysis of lymphokine mRNA expression and relevance to cancer immunotherapy. J Immunol. 1989 Jun 15;142(12):4520–4526. [PubMed] [Google Scholar]
- Chakraborty N. G., Sporn J. R., Pasquale D. R., Ergin M. T., Mukherji B. Suppression of lymphokine-activated killer cell generation by tumor-infiltrating lymphocytes. Clin Immunol Immunopathol. 1991 Jun;59(3):407–416. doi: 10.1016/0090-1229(91)90036-a. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Heo D. S., Whiteside T. L., Kanbour A., Herberman R. B. Lymphocytes infiltrating human ovarian tumors. I. Role of Leu-19 (NKH1)-positive recombinant IL-2-activated cultures of lymphocytes infiltrating human ovarian tumors. J Immunol. 1988 Jun 1;140(11):4042–4049. [PubMed] [Google Scholar]
- Kabawat S. E., Bast R. C., Jr, Welch W. R., Knapp R. C., Bhan A. K. Expression of major histocompatibility antigens and nature of inflammatory cellular infiltrate in ovarian neoplasms. Int J Cancer. 1983 Nov 15;32(5):547–554. doi: 10.1002/ijc.2910320505. [DOI] [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., Carrel S., von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986 Mar 1;136(5):1899–1907. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Moy P. M., Holmes E. C., Golub S. H. Depression of natural killer cytotoxic activity in lymphocytes infiltrating human pulmonary tumors. Cancer Res. 1985 Jan;45(1):57–60. [PubMed] [Google Scholar]
- Nitta T., Sato K., Okumura K., Steinman L. An analysis of T-cell-receptor variable-region genes in tumor-infiltrating lymphocytes within malignant tumors. Int J Cancer. 1991 Oct 21;49(4):545–550. doi: 10.1002/ijc.2910490412. [DOI] [PubMed] [Google Scholar]
- Oettgen H. F. Cytokines in clinical cancer therapy. Curr Opin Immunol. 1991 Oct;3(5):699–705. doi: 10.1016/0952-7915(91)90099-m. [DOI] [PubMed] [Google Scholar]
- Porcelli S., Brenner M. B., Band H. Biology of the human gamma delta T-cell receptor. Immunol Rev. 1991 Apr;120:137–183. doi: 10.1111/j.1600-065x.1991.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Reinhold U., Abken H., Kukel S., Goeden B., Uerlich M., Neumann U., Kreysel H. W. Tumor-infiltrating lymphocytes isolated from a Ki-1-positive large cell lymphoma of the skin. Phenotypic characterization and analysis of cytokine secretion. Cancer. 1991 Nov 15;68(10):2155–2160. doi: 10.1002/1097-0142(19911115)68:10<2155::aid-cncr2820681012>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Roszman T., Elliott L., Brooks W. Modulation of T-cell function by gliomas. Immunol Today. 1991 Oct;12(10):370–374. doi: 10.1016/0167-5699(91)90068-5. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Schwartzentruber D. J., Topalian S. L., Mancini M., Rosenberg S. A. Specific release of granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-alpha, and IFN-gamma by human tumor-infiltrating lymphocytes after autologous tumor stimulation. J Immunol. 1991 May 15;146(10):3674–3681. [PubMed] [Google Scholar]
- Takagi S., Chen K., Schwarz R., Iwatsuki S., Herberman R. B., Whiteside T. L. Functional and phenotypic analysis of tumor-infiltrating lymphocytes isolated from human primary and metastatic liver tumors and cultured in recombinant interleukin-2. Cancer. 1989 Jan 1;63(1):102–111. doi: 10.1002/1097-0142(19890101)63:1<102::aid-cncr2820630117>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Solomon D., Avis F. P., Chang A. E., Freerksen D. L., Linehan W. M., Lotze M. T., Robertson C. N., Seipp C. A., Simon P. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988 May;6(5):839–853. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Si L. S., Kanbour A., Herberman R. B., Whiteside T. L. Lymphocytes infiltrating human ovarian tumors: synergy between tumor necrosis factor alpha and interleukin 2 in the generation of CD8+ effectors from tumor-infiltrating lymphocytes. Cancer Res. 1989 Nov 1;49(21):5979–5985. [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Chan A., Chin B., Minden M., Mak T. W. Analysis of cDNA clones specific for human T cells and the alpha and beta chains of the T-cell receptor heterodimer from a human T-cell line. Proc Natl Acad Sci U S A. 1985 May;82(10):3430–3434. doi: 10.1073/pnas.82.10.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]