Abstract
Soon after the hepatitis C virus (HCV) was identified in 1989, it was recognized that the prevalence of infection in patients with ESRD far exceeded that in the general population. Infection with HCV predisposes to the hepatic complications of cirrhosis and hepatocellular carcinoma. However, important extrahepatic manifestations include immune complex glomerular disease, accelerated progression of CKD, increases in cardiovascular event risk, and lymphoproliferative disorders. Advances in understanding the molecular biology of HCV have ushered in a new era in the treatment of this infection. Second generation direct–acting antiviral agents have revolutionized therapy, with sustained virologic response rates (undetectable viral load 12 weeks after completing therapy) of >90% in most patients. Studies using direct-acting antivirals in patients with CKD and those on dialysis are showing excellent safety and efficacy as well. In this context, it is imperative that nephrologists become familiar with this literature, reviewed here, so that the important decisions, including which patients should be treated and the optimal timing to initiate therapy, are vetted in association with the compounding issues of CKD, ESRD, and kidney transplantation.
Keywords: hepatitis, kidney transplantation, chronic kidney disease
In the quarter century that has passed since hepatitis C virus (HCV) was identified,1 an extensive literature has defined the epidemiology, viral kinetics, and clinical manifestations of this infection. Soon after its discovery, HCV was recognized to be a public health issue of global significance that is estimated to affect approximately 170 million individuals worldwide,2–5 many of whom remain undiagnosed. Furthermore, it has become clear that the consequences of HCV infection extend well beyond the liver, defining more of a systemic disease with a multitude of clinical consequences.6–8
As different populations of patients were screened for anti-HCV antibodies, it became evident that the prevalence of HCV infection in patients with ESRD far exceeded that of the general population. Early reports noted prevalence rates as high as 25% in urban hemodialysis units in the United States and in excess of 50% in less developed countries. Moreover, it was unequivocally shown that HCV was transmissible by kidney transplantation (KT)9 as well as within dialysis units as a consequence of a breakdown of universal precautions.10 HCV infection was also shown to be the etiology of most cases of what had previously been referred to as essential mixed cryoglobulinemia as well as some cases of idiopathic membranoproliferative GN.7,10
Recognizing that an extensive literature had accumulated on the effect and treatment of HCV in patients with kidney disease, the first Kidney Disease Improving Global Outcomes (KDIGO) workgroup was organized in 2007 with a focus on developing clinical guidelines for the diagnosis and treatment of HCV infection.6 It was evident to the KDIGO investigators that patients with CKD and patients with ESRD had been systematically excluded from all of the phase 3 HCV treatment trials and as a consequence, that they represented a population with a substantial unmet clinical need.
This review will focus on HCV infection in patients with CKD, patients with ESRD, and patients with transplants and summarizes new information pertaining to the use of direct–acting antiviral (DAA) agents in these patients. The availability of safe and effective antiviral medications requires the development of treatment paradigms that take into account the unique needs of this patient population.
The HCV
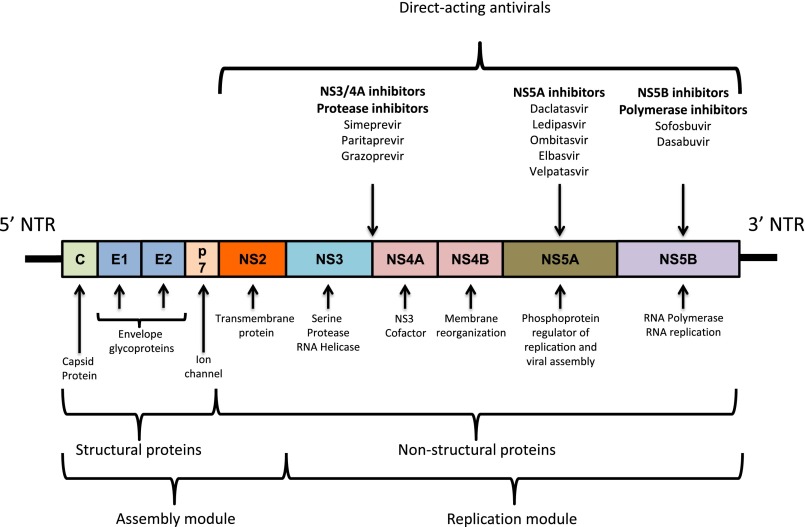
HCV is a small (50 nm) enveloped virus that was first isolated and cloned in 1989.1 It has a positive single–stranded RNA with approximately 9600 nucleotides11 and a genome composed of structural and nonstructural proteins (Figure 1).
Figure 1.
The HCV genome and target sites of action for the direct acting antiviral agents. Combining agents with different mechanisms of viral interference has resulted in the achievement of very high sustained viral response rates. NTR, nontranslated region.
Seven genotypes have been identified, with each divided into subtypes and strains.12,13 Genotypes 1–3 are distributed globally, with 1a and 1b being the most common (60% of the infections worldwide). Genotype 1a is the dominant genotype in northern Europe and North America, whereas 1b is distributed worldwide. In contrast, genotypes 2 (Europe and Mediterranean region), 3 (Asia), 4 (Middle East and central Africa), 5 (South Africa), 6 (Asia) and 7 (Central Africa) have a penetration that is more geographically specific.11,13
HCV in Patients with Kidney Disease
HCV and CKD
Patients with CKD have a higher prevalence of HCV infection compared with the general population.10 Recent studies suggest that HCV-infected patients with CKD have an accelerated rate of kidney function loss and an increased risk of progressing to ESRD.14–19 In one study, Molnar et al.19 showed that HCV-infected patients with CKD had an increased mortality and an accelerated rate of progression to ESRD, raising the important question of whether treating to obtain a sustained virologic response defined as an undetectable viral load 12 weeks after completion of treatment (SVR12) would diminish the rate of decline in GFR.
Infection with HCV has been implicated as being causative in most patients with mixed cryoglobulinemia and several histologic forms of glomerular injury, including membranoproliferative and membranous GN.7,20 Patients coinfected with HIV have been reported to have an increased mortality and overall worse prognosis.21,22
HCV in the Patient with ESRD
The prevalence of HCV in patients with ESRD has always exceeded that of the general population, and transmission of the virus within dialysis clinics has been unequivocally shown.10 The risk of transmission is an ongoing hazard as emphasized in a recent Centers for Disease Control and Prevention (CDC) health advisory that noted an increased number of acute HCV infection events among patients undergoing maintenance hemodialysis.23 Lapses in universal precautions were identified at these dialysis clinics, emphasizing the need for continuously improving infection control practices and environmental disinfection procedures accompanied by adherence to recommended CDC HCV screening protocols.24
Studies have consistently shown that HCV infection is associated with an increased mortality in patients with ESRD.25–29 In a meta-analysis by Fabrizi et al.,26 the relative risk of mortality was 1.35 (95% confidence interval, 1.25 to 1.47) among HCV-infected patients with ESRD. Analysis of cause-specific death showed an increased burden of cardiovascular risk, supporting the concept that HCV is a systemic disease with important extrahepatic consequences.26 Of interest, transplanting an anti-HCV–positive patient with ESRD is accompanied by a significantly decreased risk of death compared with remaining on the waiting list. This survival benefit is largely attributable to a decrease in cardiovascular events within the first year post-transplant.30 Thus, although it is important to recognize that HCV–infected kidney recipients have an increased hazard ratio for death, transplant continues to offer these patients a significantly improved survival compared with remaining on dialysis.25–29,31
HCV and KT
HCV infection is the primary cause of liver disease in KT recipients32; however, it has also been associated with important extrahepatic manifestations that contribute to increased morbidity and mortality after transplantation.33 HCV-infected recipients are at increased risk of de novo and recurrent membranous nephropathy, membranoproliferative GN,34–36 and transplant glomerulopathy.37 Infection with HCV has also been associated with an increased risk for insulin resistance and diabetes mellitus in waitlisted candidates and kidney recipients, similar to that reported for the general population.38–43 Although prospective studies are lacking, it is reasonable to assume that the increased incidence of diabetes in the HCV–infected KT candidate/recipient would translate into an increased burden of cardiovascular event risk.
The presence of anti-HCV antibody is associated with lower patient and graft survival among KT recipients.44,45 In a large meta-analysis, Fabrizi et al.44 showed a significant increase in both mortality and graft loss among HCV-infected recipients. However, in a retrospective study of 230 patients, Roth et al.31 showed that KT in HCV-infected patients conferred a long–term survival benefit compared with remaining on the waitlist. Thus, chronic HCV infection should not be considered a contraindication to KT, because the long–term survival advantage associated with transplantation can still be shown in these patients.31,46–49
DAA Agents in Patients with CKD
Patients achieving an SVR12 are considered cured of the infection. Using the latest generation DAAs, rates of SVR12 in clinical trials conducted in the general population exceed 90% across all genotypes, with the lowest response rates being reported in cirrhotics and patients with genotype 3. The evolution from IFN-based therapy to DAAs is a remarkable accomplishment of the last decade and offers the possibility of life-saving treatment for millions of patients.50,51
DAAs
Sofosbuvir
Sofosbuvir is a nucleotide analog NS5B polymerase inhibitor that targets the nonstructural protein 5B RNA–dependent RNA polymerase, thus effectively terminating viral replication (Figure 1, Table 1). Sofosbuvir is a prodrug that is phosphorylated into the active metabolite GS-461203, with subsequent dephosphorylation into the inactive metabolite GS-331007.52 Sofosbuvir and GS-331007 are cleared by the kidneys, resulting in significant accumulation of both in patients with CKD.53 In this context, the safety and efficacy of sofosbuvir in patients with advanced CKD or ESRD have not been established, and the drug is not recommended for use in patients with a creatinine clearance of <30 ml/min. Nevertheless, two recent studies using open-label treatment with simeprevir and dose-adjusted sofosbuvir in patients with advanced CKD/ESRD showed safety and high rates of SVR12.54,55 Additional experience with sofosbuvir in HCV-infected patients with CKD has been reported from the HCV-TARGET Study, a longitudinal real world observational study of DAAs.56 SVR12 rates of 85%–90% were achieved across all patient groups; however, increased adverse events were reported in patients with more advanced kidney disease (eGFR<45 ml/min). Current guidelines from the American Association for the Study of Liver Disease (AASLD) recommend that sofosbuvir only be considered as an option for patients with creatinine clearance of <30 ml/min when expert opinion is obtained before initiating therapy.57
Table 1.
DAA agents
| Medication | Dose | Clearance | Target of Action | Use in CKD Stages 4 and 5 |
|---|---|---|---|---|
| Sofosbuvir | 400 mg daily | Renal =81%; GI=15% | NS5B | eGFR=15–29 ml/min: not recommended; eGFR<15 ml/min: not recommended; limited data available |
| Simeprevir | 150 mg daily | Renal <1%; GI=91% | NS3A/4A | eGFR=15–29 ml/min: dose adjustment not required; eGFR<15 ml/min: not recommended; limited data available |
| Ledipasvir | 90 mg daily | Renal =1%; GI=86% | NS5A | eGFR=15–29 ml/min: dose adjustment not required; eGFR<15 ml/min: not recommended |
| Ombitasvir/paritaprevir/ritonavir; dasabuvir | 12.5/75/50 mg ×2 tablets; 250 mg ×2 tablets | Renal <2%; GI=90% | NS5A/NS3A/4A/CYP3A; NS5B | eGFR=15–29 ml/min: dose adjustment not required; eGFR<15 ml/min: dose adjustment not required; not studied in patients on dialysis; limited data |
| Grazoprevir/elbasvir | 100/50 mg daily | Renal <1% | NS3/4A NS5A | eGFR=15–29 ml/min: dose adjustment not required; eGFR<15 ml/min: dose adjustment not required; dialysis population studied |
| Daclatasvir | 60 mg daily; used with sofosbuvir | Renal =7%; GI=88% | NS5A | eGFR=15–29 ml/min: dose adjustment not required; eGFR<15 ml/min: dose adjustment not required |
GI, gastrointestinal.
Simeprevir
Simeprevir is a nonstructural protein 3/4A protease inhibitor that is metabolized in the liver (>90%) with insignificant renal clearance (<1%). The drug is highly protein bound, and it is not cleared by dialysis; thus, no dosing adjustments are required for patients with CKD or ESRD.58 Limited data are available on the safety and efficacy of simeprevir in the CKD and ESRD population. Bhamidimarri et al.54 reported the use of full-dose simeprevir combined with half-dose sofosbuvir in patients with advanced CKD/ESRD. This combination was found to be well tolerated, with limited adverse events and excellent efficacy.
Ledipasvir
Ledipasvir is a nonstructural protein 5A inhibitor (NS5A) that is marketed in combination with sofosbuvir (Harvoni).59,60 The drug is hepatically metabolized with minimal renal clearance, and as a consequence, the AASLD does not recommend dose adjustments for patients with CKD or ESRD.57 However, because it is prepared in combination with sofosbuvir, caution must be used in patients with GFR<30 ml/min.
Ombitasvir-Paritaprevir-Ritonavir and Dasabuvir (3D Regimen)
The combination of ombitasvir/paritaprevir (ritonavir boosted) and dasabuvir is marketed as Viekira Pak. Ombitasvir is an inhibitor of the nonstructural protein 5A (NS5A), whereas paritaprevir is a nonstructural protein 3/4A protease inhibitor that is combined with low-dose ritonavir to boost paritaprevir drug levels.61 These three drugs are administered with dasabuvir, an inhibitor of the nonstructural protein 5B RNA–dependent RNA polymerase (NS5B). This combination was studied in the RUBY-I Trial, a multicenter, phase 3b study assessing the safety and efficacy of the three-dimensional regimen in patients with CKD stage 4/5.62 In preliminary reports, 17 of 17 patients achieved an SVR12, and no treatment–related serious adverse events were noted. No dose adjustments of ombitasvir-paritaprevir/ritonavir and dasabuvir are required in patients with advanced CKD; however, insufficient data are available to recommend their use in patients on dialysis.
Daclatasvir
Daclatasvir is a nonstructural protein 5A (NS5A) inhibitor that blocks both RNA replication and virion assembly. As a consequence of the drugs hepatic metabolism, no dosing adjustments are necessary in patients with CKD.63 Daclatasvir has been used mostly in combination with sofosbuvir for patients with genotype 3 infection.64 In a study including patients with mild to moderate CKD and ESRD, daclatasvir was well tolerated with good efficacy.65
Grazoprevir and Elbasvir
Grazoprevir is a nonstructural protein 3/4A protease inhibitor (NS3/4A) that has been combined with the nonstructural protein 5A (NS5A) inhibitor elbasvir. This product (Zepatier) recently received Food and Drug Administration approval for the treatment of HCV genotypes 1 and 4 infection and should be available in the United States within several months. Both grazoprevir and elbasvir are highly protein bound and thus, not cleared by dialysis.66 Additionally, they rely on hepatic metabolism and do not accumulate in patients with reduced kidney function. This combination was studied in the C-SURFER Trial, the first prospective, randomized study of DAAs that focused exclusively on HCV-infected patients with CKD and HCV-infected patients with ESRD.67 In this phase 3 trial, 224 patients with HCV genotype 1 infection and stage 4 or 5/5D CKD were randomly assigned to an immediate treatment group (n=111) or a deferred treatment group (n=113) that initially received placebo and then, was offered active therapy. Data from an intensive pharmacokinetic group (n=11) showed no requirement for dose adjustment in patients on hemodialysis. Treatment with Zepatier resulted in SVR12s of 99%, with a low adverse event profile and no significant safety signals in this CKD population.67,68
Velpatasvir
Velpatasvir is a nonstructural protein 5A (NS5A) inhibitor with antiviral activity against genotypes 1–6.69 Two studies have shown that velpatasvir combined with sofosbuvir provided high rates of SVR12 in patients with all genotypes, including the difficult to treat patients with genotype 3.70,71 As has been the case in most pivotal trials of HCV infection in the general population, patients with kidney disease were not included in these studies; thus, there are no data on the safety and efficacy of this combination in patients with CKD and patients with ESRD.71
Special Considerations
HCV Infection in the KT Candidate
All patients being evaluated for KT should be screened for HCV infection. The KDIGO guidelines recommended ELISA testing in geographic regions with a low prevalence of HCV followed with nucleic acid testing confirmation of all positive results. Initial testing with nucleic acid testing was recommended in regions with a high prevalence of HCV.6 An accurate assessment of the extent of liver injury is necessary in all KT candidates with active viral replication. Liver biopsy has always been considered the gold standard; however, there are now several noninvasive tests that have shown promise in making this determination. These methods rely on distinct but complementary approaches: a biologic method that quantifies serum levels of biomarkers of fibrosis and a physical approach that measures liver stiffness by ultrasound or magnetic resonance elastography (FibroTest and FibroScan).72 Validation of these surrogate markers of liver fibrosis in the patient with ESRD will be needed. A liver biopsy should be considered in inconclusive cases or if there is a suggestion of advanced fibrosis (stage 3/4) on the noninvasive testing.
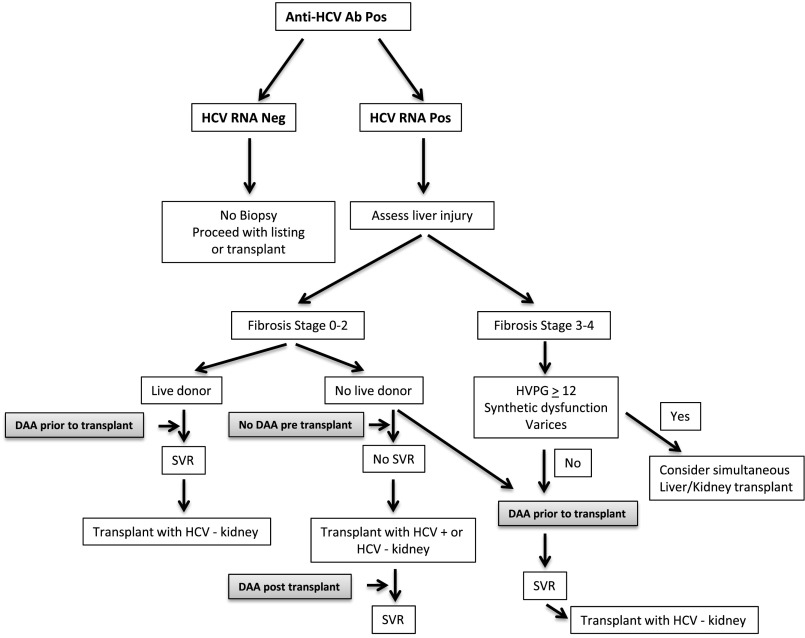
We have been using a management paradigm73,74 (Figure 2) similar to the one proposed by Sawinski et al.73 Patients with METAVIR stages 0–2 liver fibrosis who have a living donor should receive DAAs before transplant to achieve SVR12. For the patient without a living donor, there are two options available at the time of listing. In one scenario, the patient can delay DAA treatment (thus remaining viremic), with the intention of receiving a kidney from an HCV-positive donor. Of note, this option is not available at all transplant centers, and an informed consent should be obtained. This approach has been accompanied by significantly reduced waiting times compared with those for HCV-negative kidneys.75 In this scenario, DAA therapy can be initiated in the post-transplant period. We would recommend waiting 2–3 months post-transplant to achieve stable kidney function and immunosuppressive dosing (see below). The primary objective of transplanting a kidney from an HCV-positive donor would be to shorten waiting times and make transplantation more available by using kidneys that are often discarded.76 If this option was not available, the patient would be confronted with a waiting times that can often exceed 5 years. In this case, consideration should be given to pretransplant treatment with DAAs to achieve an SVR12, thus limiting the risk of progressive liver injury. In this scenario, the patient can wait to receive a kidney from an uninfected donor or consider transplantation of a kidney from an HCV-infected donor, with early initiation of DAAs in the post-transplant period. In a similar context, there is increased interest in transplanting kidneys from positive donors into negative recipients followed by early initiation of DAAs post-transplant. Reese et al.76 discussed this perspective in a recent editorial and propose this as a reasonable consideration for uninfected patients with high risk of health deterioration because of dialysis (elderly, cardiovascular morbidity, or access failure) or those with disadvantageous blood types. Any decision in this regard must be balanced against data suggesting that uninfected patients that receive HCV-positive kidneys have increased mortality.77 Furthermore, the ethics of knowingly infecting a patient with HCV must be carefully weighed against the knowledge that not all patients achieve an SVR with DAA treatment, and there is no certainty that payers will approve this costly therapy after transplantation.
Figure 2.
Suggested paradigm for the management of the HCV-infected pretransplant candidate. Critical decision points include whether the patient has a living donor and the extent of liver fibrosis. The option to accept a kidney from a HCV positive donor could significantly shorten wait times. HVPG, hepatic venous pressure gradient; Neg, negative; Pos, positive; SVR, sustained virologic response; DAA, direct antiviral agent.
Any discussion of knowingly infecting a patient with HCV at the time of transplant must be with full understanding of the accompanying risk of fibrosing cholestatic hepatitis, an aggressive form of HCV seen after liver transplantation or KT during the period of maximal immunosuppression.78 This was a serious concern during the IFN/ribavirin era when SVRs were low, and the treatment was associated with an increased risk of renal allograft rejection.79 These concerns may be diminished by recent data from liver recipients showing successful treatment of fibrosing cholestatic hepatitis using current DAAs.80–83 These results are encouraging; however, we await confirmation of these outcomes in kidney recipients.
The second option for the HCV–infected transplant candidate would be to treat with DAAs before transplant. As mentioned earlier, this would be a reasonable plan for the patient with a living donor. This might also be an advisable approach for the patient with more advanced histologic liver injury (stage 3/4 liver disease with early cirrhosis) and without a living donor. These patients must be carefully evaluated to determine if a kidney-alone transplant is advisable or if a combined liver transplant/KT is necessary. Much of this decision will hinge on whether the patient has preserved synthetic liver function and/or portal hypertension.74 At our center, these patients have transjugular measurement of hepatic venous pressure gradient. Patients with a gradient ≤12 mmHg with normal platelet count and synthetic liver function are treated with DAAs to achieve an SVR12 and subsequently, listed to receive a kidney from an HCV-negative donor. This will require the usual waiting times on the list; however, it seems prudent not to transplant a cirrhotic patient with active viral replication. Each of these options must be carefully reviewed with the patient, because the treatment strategy will have a significant effect on the patient’s clinical course.73,76 Furthermore, the decision to use a hepatic venous pressure gradient of ≤12 as a component of this decision is not on the basis of prospective studies and should not to be considered the standard of care. Successfully establishing a paradigm for the use of kidneys from anti-HCV–positive donors will likely increase the number of patients transplanted by using kidneys that are currently being discarded and/or not being harvested.76,84
HCV Treatment in the KT Recipient
For the last two decades, the treatment of HCV infection in the post-transplant setting has not been an option in large part because of the unacceptable risk of rejection associated with the use of IFN-based regimens.84–87 Furthermore, the IFNs are poorly tolerated, and SVR rates are only in the 45%–50% range.88 As a consequence of this poor efficacy and high adverse event profile, treatment of HCV infection in the KT candidate and recipient has often been deferred.
In the era of DAAs, there are several important caveats when opting to treat post-transplantation. There are potential drug-drug interactions that must be considered. Both the calcineurin inhibitors and mammalian target of rapamycin inhibitors are substrates of cytochrome p450 isoenzymes 3A4/5 and the drug transporter, P-glycoprotein (Table 2). Although sofosbuvir and/or daclatasvir do not interact with cytochrome p450 3A4/5 or P-glycoprotein, simeprevir, ledispasvir, and Vikiera Pak should be used with caution to avoid sub- or supratherapeutic immunosuppression. Recently reported studies from KT recipients have shown the need to adjust tacrolimus dosing in patients receiving DAAs.89,90
Table 2.
Use of DAAs with immunosuppressive agents
| Immunosuppressant | SOF and LDV | OMV, PTV, r, and DSV | SMV | SOF |
|---|---|---|---|---|
| Cyclosporin | No changes in levels | ↑ Cyclosporin levels (r) | ↑ Levels of both cyclosporin and SMV | No changes in levels |
| Tacrolimus | No changes in levels | ↑ Tacrolimus levels (r) | ↓ Tacrolimus levels; monitor levels closely | No changes in levels |
| Sirolimus | No changes in levels | ↑ Sirolimus levels (r) | ↑ or ↓ Levels of sirolimus | No changes in levels |
SOF, sofosbuvir; LDV, ledipasvir; OMV, ombitasvir; PTV, paritaprevir; r, ritonavir; DSV, dasabuvir; SMV, simeprevir.
With the availability of DAA agents that are safe and effective in transplant recipients, the important decisions will now focus on determining the safest and most effective combination of DAAs coordinated with the optimal timing of therapy.89–91 In this context, nephrologists must be at the forefront of these decisions, because they will have an enormous effect on the timing of transplantation. Furthermore, treatment of the post-transplant patient mandates very careful monitoring of kidney function and immunosuppressive drug levels to ensure the maintenance of adequate immunosuppression.92,93
Conclusion
HCV infection is a global public health issue with important hepatic and extrahepatic manifestations. Although patients with CKD had been largely excluded from most phase 3 DAA trials, data are now becoming available that raise hopes for a cure in the patient with ESRD and the KT recipient. In this context, it will be essential that nephrologists become familiar with the different DAAs and their drug-drug interactions and provide guidance on the optimal timing for the initiation of therapy. Patients with kidney disease and HCV infection have waited decades for this opportunity, and as is often the case with other comorbidities, they will expect the nephrologist to orchestrate the nuances of the treatment decisions that will be necessary.
Disclosures
M.L. and F.P. have no financial disclosures. D.R. has been a consultant for Merck GmbH (Darmstadt, Germany) and Abbvie and served on a Scientific Advisory Board for Merck GmbH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Farci P: Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome [Science 1989;244:359-362]. J Hepatol 36: 582–585, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bunchorntavakul C, Maneerattanaporn M, Chavalitdhamrong D: Management of patients with hepatitis C infection and renal disease. World J Hepatol 7: 213–225, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwo PY, Agrawal S: HCV/HIV coinfection: A new treatment paradigm. Gastroenterology 148: 1470–1471, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Marinaki S, Boletis JN, Sakellariou S, Delladetsima IK: Hepatitis C in hemodialysis patients. World J Hepatol 7: 548–558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GL, Albright JE, Cook SF, Rosenberg DM: Projecting future complications of chronic hepatitis C in the United States. Liver Transpl 9: 331–338, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO): Clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 109: S1–S99, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE, Wilson R: Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 328: 465–470, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Morales JM, Kamar N, Rostaing L: Hepatitis C and renal disease: Epidemiology, diagnosis, pathogenesis and therapy. Contrib Nephrol 176: 10–23, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Pereira BJ, Milford EL, Kirkman RL, Levey AS: Transmission of hepatitis C virus by organ transplantation. N Engl J Med 325: 454–460, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Martin P, Fabrizi F: Hepatitis C virus and kidney disease. J Hepatol 49: 613–624, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Jang JY, Chung RT: Chronic hepatitis C. Gut Liver 5: 117–132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano T, Lau GM, Lau GM, Sugiyama M, Mizokami M: An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region. Liver Int 32: 339–345, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Murphy DG, Sablon E, Chamberland J, Fournier E, Dandavino R, Tremblay CL: Hepatitis C virus genotype 7, a new genotype originating from central Africa. J Clin Microbiol 53: 967–972, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park H, Adeyemi A, Henry L, Stepanova M, Younossi Z: A meta-analytic assessment of the risk of chronic kidney disease in patients with chronic hepatitis C virus infection. J Viral Hepat 22: 897–905, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, Yu ML, Hwang SJ: Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One 9: e100790, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YC, Chiou WY, Hung SK, Su YC, Hwang SJ: Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: A 6-year nationwide cohort study across Taiwan. BMC Nephrol 14: 187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YC, Lin HY, Li CY, Lee MS, Su YC: A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int 85: 1200–1207, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Fabrizi F, Messa P, Martin P: Recent advances on hepatitis C virus in dialysis population. Kidney Blood Press Res 39: 260–271, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, Kovesdy CP: Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology 61: 1495–1502, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansonno D, Gesualdo L, Manno C, Schena FP, Dammacco F: Hepatitis C virus-related proteins in kidney tissue from hepatitis C virus-infected patients with cryoglobulinemic membranoproliferative glomerulonephritis. Hepatology 25: 1237–1244, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Scherzer R, Shlipak MG: Risk factors: Individual assessment of CKD risk in HIV-positive patients. Nat Rev Nephrol 11: 392–393, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Klein MB, Rollet-Kurhajec KC, Moodie EE, Yaphe S, Tyndall M, Walmsley S, Gill J, Martel-Laferriere V, Cooper C; Canadian Co-infection Cohort Investigators : Mortality in HIV-hepatitis C co-infected patients in Canada compared to the general Canadian population (2003-2013). AIDS 28: 1957–1965, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Available at: http://emergency.cdc.gov/han/han00386.asp. Accessed February 17, 2016
- 24.Bailey J, Balter P, Berns J, Butera E, Depner T, Douglas C, Duncan E, Edwards B, Favero M, Foreman J, Fried M, Gandi V, Glynn C, Hakim R, Heard N, Lazaruz J, Levin N, Light P, Lindefield P, Masrshall C, Matyas B, Mazilli J, McCool B, Meisels I, Meyers C, Natov S, Nutter C, Pastan S, Peacock E, Polder J, Sharbaugh R, Shimojura G, Steinber J, Thomas-Hawkins C, Vas S, Walters D, Weber D, Wingard R, Wish J: Consultant Meeting to Update Recommendations for the Prevention and Control of Bloodborne and Other Infections Among Chronic Hemodialysis Patients, 1999. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5005a1.htm. Accessed February 17, 2016
- 25.Ingsathit A, Kamanamool N, Thakkinstian A, Sumethkul V: Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: A systematic review and meta-analysis. Transplantation 95: 943–948, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Fabrizi F, Dixit V, Messa P: Impact of hepatitis C on survival in dialysis patients: A link with cardiovascular mortality? J Viral Hepat 19: 601–607, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P: The impact of hepatitis C virus infection on survival in dialysis patients: Meta-analysis of observational studies. J Viral Hepat 14: 697–703, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Fabrizi F, Ganeshan SV, Lunghi G, Messa P, Martin P: Antiviral therapy of hepatitis C in chronic kidney diseases: Meta-analysis of controlled clinical trials. J Viral Hepat 15: 600–606, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Miller LG, Daar ES, Gjertson DW, Kopple JD, Greenland S: Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 18: 1584–1593, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Briggs JD: Causes of death after renal transplantation. Nephrol Dial Transplant 16: 1545–1549, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Roth D, Gaynor JJ, Reddy KR, Ciancio G, Sageshima J, Kupin W, Guerra G, Chen L, Burke GW 3rd: Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol 22: 1152–1160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baid-Agrawal S, Pascual M, Moradpour D, Frei U, Tolkoff-Rubin N: Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev Med Virol 18: 97–115, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Baid-Agrawal S, Pascual M, Moradpour D, Somasundaram R, Muche M: Hepatitis C virus infection and kidney transplantation in 2014: What’s new? Am J Transplant 14: 2206–2220, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Morales JM, Pascual-Capdevila J, Campistol JM, Fernandez-Zatarain G, Muñoz MA, Andres A, Praga M, Martinez MA, Usera G, Fuertes A, Oppenheimer F, Artal P, Darnell A, Rodicio JL: Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation 63: 1634–1639, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Cruzado JM, Carrera M, Torras J, Grinyó JM: Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant 1: 171–178, 2001 [PubMed] [Google Scholar]
- 36.Roth D, Cirocco R, Zucker K, Ruiz P, Viciana A, Burke G, Carreno M, Esquenazi V, Miller J: De novo membranoproliferative glomerulonephritis in hepatitis C virus-infected renal allograft recipients. Transplantation 59: 1676–1682, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Baid-Agrawal S, Farris AB 3rd, Pascual M, Mauiyyedi S, Farrell ML, Tolkoff-Rubin N, Collins AB, Frei U, Colvin RB: Overlapping pathways to transplant glomerulopathy: Chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int 80: 879–885, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Milner KL, van der Poorten D, Trenell M, Jenkins AB, Xu A, Smythe G, Dore GJ, Zekry A, Weltman M, Fragomeli V, George J, Chisholm DJ: Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology 138: 932–941, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL: Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med 133: 592–599, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G: Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: Meta-analysis of clinical studies. Am J Transplant 5: 2433–2440, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Delgado-Borrego A, Casson D, Schoenfeld D, Somsouk M, Terella A, Jordan SH, Bhan A, Baid S, Cosimi AB, Pascual M, Chung RT: Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation 77: 703–710, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Baid-Agrawal S, Frei U, Reinke P, Schindler R, Kopp MA, Martus P, Berg T, Juergensen JS, Anker SD, Doehner W: Impaired insulin sensitivity as an underlying mechanism linking hepatitis C and posttransplant diabetes mellitus in kidney recipients. Am J Transplant 9: 2777–2784, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Naing C, Mak JW, Wai N, Maung M: Diabetes and infections-hepatitis C: Is there type 2 diabetes excess in hepatitis C infection? Curr Diab Rep 13: 428–434, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Fabrizi F, Martin P, Dixit V, Messa P: Meta-analysis of observational studies: Hepatitis C and survival after renal transplant. J Viral Hepat 21: 314–324, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Morales JM, Bloom R, Roth D: Kidney transplantation in the patient with hepatitis C virus infection. Contrib Nephrol 176: 77–86, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Sezer S, Ozdemir FN, Akcay A, Arat Z, Boyacioglu S, Haberal M: Renal transplantation offers a better survival in HCV-infected ESRD patients. Clin Transplant 18: 619–623, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Knoll GA, Tankersley MR, Lee JY, Julian BA, Curtis JJ: The impact of renal transplantation on survival in hepatitis C-positive end-stage renal disease patients. Am J Kidney Dis 29: 608–614, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Pereira BJ, Natov SN, Bouthot BA, Murthy BV, Ruthazer R, Schmid CH, Levey AS; The New England Organ Bank Hepatitis C Study Group : Effects of hepatitis C infection and renal transplantation on survival in end-stage renal disease. Kidney Int 53: 1374–1381, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Bloom RD, Sayer G, Fa K, Constantinescu S, Abt P, Reddy KR: Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant 5: 139–144, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Heim MH: 25 years of interferon-based treatment of chronic hepatitis C: An epoch coming to an end. Nat Rev Immunol 13: 535–542, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Maruyama A, Partovi N, Yoshida EM, Erb SR, Azalgara VM, Hussaini T: A review of direct-acting antivirals for the treatment of hepatitis C in patients with advanced chronic kidney disease [published online ahead of print October 19, 2015]. Nephrol Dial Transplant [DOI] [PubMed] [Google Scholar]
- 52.Gilead Sciences, Inc.: Sovaldi 400 mg Tablets (Sofosbuvir) Product Monograph, 2014. Available at: http://www.gilead.ca/pdf/ca/sovaldi_pm_english.pdf. Accessed November 15, 2015
- 53.Kirby BJ, Symonds WT, Kearney BP, Mathias AA: Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin Pharmacokinet 54: 677–690, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Bhamidimarri KR, Czul F, Peyton A, Levy C, Hernandez M, Jeffers L, Roth D, Schiff E, O’Brien C, Martin P: Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol 63: 763–765, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Sabucedo A, Antoine M, Jorge D, Andreu A, Pedraza F, Hernandez M, Jeffers L, Ladino M: Sofosbuvir use in patients with Hepatitis C virus infection and severe chronic kidney disease [Abstract]. J Am Soc Nephrol 26: 663A, 2015. 25071082 [Google Scholar]
- 56.Saxena V, Koraishy FM, Sise ME, Lim JK, Schmidt M, Chung RT, Liapakis A, Nelson DR, Fried MW, Terrault N: HCV-TARGET: Safety and efficacy of sofosbuvir-containing regimens in HCV infected patients with impaired renal function [published online ahead of print February 29, 2016]. Liver Int doi: 10.1111/liv.13102 [DOI] [PMC free article] [PubMed]
- 57.American Association for the Study of Liver Diseases and the Infectious Diseases Society of America: HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Unique Patient Populations: Patients with Renal Impairment, 2015. Available at: http://www.hcvguidelines.org/full-report/unique-patient-populations-patients-renal-impairment. Accessed November 15, 2015
- 58.Janssen, Inc.: Galexos (Simeprevir) Product Monograph, 2015. Available at: http://www.janssen.ca/product/575. Accessed November 15, 2015
- 59.Gilead Sciences, Inc.: HARVONI (Ledipasvir/Sofosbuvir 90 mg/400 mg) Product Monograph, 2014. Available at: http://www.gilead.ca/pdf/ca/harvoni_pm_french.pdf. Accessed November 15, 2015
- 60.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, Foster GR, Bräu N, Buti M, Jacobson IM, Subramanian GM, Ding X, Mo H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Mangia A, Marcellin P; ION-1 Investigators : Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370: 1889–1898, 2014 [DOI] [PubMed] [Google Scholar]
- 61.AbbVie Inc.: Viekira Pak [Package Insert]. Available at: http://www.viekirahcp.com/dosing/mechanism-of-action/, http://www.viekirahcp.com/dosing/regimen/. Accessed November 15, 2015
- 62.Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, Bernstein D, Podsadecki T, Cohen D, Shulman NS: Safety of ombitasvir/paritaprevir/ritonavir plus dasabuvir for treating HCV G1 infection in patients with severe renal impairment or end‐ stage renal disease: The RUBY‐1 study [Abstract]. J Hepatol 62: S257, 2015 [Google Scholar]
- 63.Bristol-Myers Squibb Company: Daklinza [Package Insert]. Available at: http://www.daklinzahcp.bmscustomerconnect.com. Accessed November 15, 2015
- 64.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R, Oguchi G, Thuluvath PJ, Ortiz-Lasanta G, Rabinovitz M, Bernstein D, Bennett M, Hawkins T, Ravendhran N, Sheikh AM, Varunok P, Kowdley KV, Hennicken D, McPhee F, Rana K, Hughes EA; ALLY-3 Study Team : All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 61: 1127–1135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garimella T, Wang R, Luo WL, Hwang C, Sherman D, Kandoussi H, Marbury TC, Alcorn H, Bertz R, Bifano M: Single-dose pharmacokinetics and safety of daclatasvir in subjects with renal function impairment. Antivir Ther 20: 535–543, 2015 [DOI] [PubMed] [Google Scholar]
- 66.Yeh W, Caro L, Guo Z, Hwa-Ping F, Davis H, Kozisek M, Stypinski D, Fen C, Mitchell C, Gillespie A, Ichhpurani N, Marshall W, Lasseter K, Marbury T, Butterton J: Pharmacokinetics of co-administered HCV protease inhibitor grazoprevir (MK-5172) and NS5A elbasivr (MK-8742) in volunteers with end-stage renal disease on hemodialysis or severe renal impairment not on hemodialysis [Abstract]. J Hepatol 60[Suppl 4]: 1940, 2014 [Google Scholar]
- 67.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr., Martin P, Pol S, Londoño MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W: Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): A combination phase 3 study. Lancet 386: 1537–1545, 2015 [DOI] [PubMed] [Google Scholar]
- 68.Chan HL, Li PK: Chronic kidney disease: Treatment of hepatitis C virus infection in patients with CKD. Nat Rev Nephrol 12: 5–6, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Lawitz E, Freilich B, Link J, German P, Mo H, Han L, Brainard DM, McNally J, Marbury T, Rodriguez-Torres M: A phase 1, randomized, dose-ranging study of GS-5816, a once-daily NS5A inhibitor, in patients with genotype 1-4 hepatitis C virus. J Viral Hepat 22: 1011–1019, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL, Mazzotta F, Moreno C, Yoshida E, Shafran SD, Towner WJ, Tran TT, McNally J, Osinusi A, Svarovskaia E, Zhu Y, Brainard DM, McHutchison JG, Agarwal K, Zeuzem S; ASTRAL-1 Investigators : Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 373: 2599–2607, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourlière M, Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H, Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM, McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia A, Sulkowski M; ASTRAL-2 Investigators; ASTRAL-3 Investigators : Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 373: 2608–2617, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Castera L: Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 142: 1293–1302.e4, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Sawinski D, Bloom RD: Novel Hepatitis C treatments and the impact on kidney transplantation. Transplantation 99: 2458–2466, 2015 [DOI] [PubMed] [Google Scholar]
- 74.Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD: Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK). Am J Transplant 8: 2243–2251, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL: Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant 10: 1238–1246, 2010 [DOI] [PubMed] [Google Scholar]
- 76.Reese PP, Abt PL, Blumberg EA, Goldberg DS: Transplanting hepatitis C-positive kidneys. N Engl J Med 373: 303–305, 2015 [DOI] [PubMed] [Google Scholar]
- 77.Abbott KC, Bucci JR, Matsumoto CS, Swanson SJ, Agodoa LY, Holtzmuller KC, Cruess DF, Peters TG: Hepatitis C and renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol 14: 2908–2918, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Delladetsima JK, Boletis JN, Makris F, Psichogiou M, Kostakis A, Hatzakis A: Fibrosing cholestatic hepatitis in renal transplant recipients with hepatitis C virus infection. Liver Transpl Surg 5: 294–300, 1999 [DOI] [PubMed] [Google Scholar]
- 79.Althaf MM, Abdelsalam MS, Rashwan M, Nadri Q: Acute hepatitis C infection in a renal transplant recipient: Primacy of the liver or kidney? BMJ Case Rep 2014: bcr2014203530, 2014 [DOI] [PMC free article] [PubMed]
- 80.Issa D, Eghtesad B, Zein NN, Yerian L, Cruise M, Alkhouri N, Adams R, Hanouneh IA: Sofosbuvir and simeprevir for the treatment of recurrent hepatitis C with fibrosing cholestatic hepatitis after liver transplantation. Int J Organ Transplant Med 7: 38–45, 2016 [PMC free article] [PubMed] [Google Scholar]
- 81.Saab S, Jimenez M, Bau S, Goo T, Zhao D, Durazo F, Han S, El Kabany M, Kaldas F, Tong MJ, Busuttil RW: Treating fibrosing cholestatic hepatitis C with sofosbuvir and ribavirin: A matched analysis. Clin Transplant 29: 813–819, 2015 [DOI] [PubMed] [Google Scholar]
- 82.Forns X, Charlton M, Denning J, McHutchison JG, Symonds WT, Brainard D, Brandt-Sarif T, Chang P, Kivett V, Castells L, Prieto M, Fontana RJ, Baumert TF, Coilly A, Londoño MC, Habersetzer F: Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology 61: 1485–1494, 2015 [DOI] [PubMed] [Google Scholar]
- 83.Delabaudière C, Lavayssière L, Dörr G, Muscari F, Danjoux M, Sallusto F, Peron JM, Bureau C, Rostaing L, Izopet J, Kamar N: Successful treatment of fibrosing cholestatic hepatitis with pegylated interferon, ribavirin and sofosbuvir after a combined kidney-liver transplantation. Transpl Int 28: 255–258, 2015 [DOI] [PubMed] [Google Scholar]
- 84.Coilly A, Samuel D: Pros and cons: Usage of organs from donors infected with hepatitis C virus - Revision in the direct-acting antiviral era. J Hepatol 64: 226–231, 2016 [DOI] [PubMed] [Google Scholar]
- 85.Morales JM, Fabrizi F: Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol 11: 172–182, 2015 [DOI] [PubMed] [Google Scholar]
- 86.Ozgür O, Boyacioğlu S, Telatar H, Haberal M: Recombinant alpha-interferon in renal allograft recipients with chronic hepatitis C. Nephrol Dial Transplant 10: 2104–2106, 1995 [PubMed] [Google Scholar]
- 87.Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D: Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation 59: 1426–1431, 1995 [DOI] [PubMed] [Google Scholar]
- 88.Rosen HR: Clinical practice. Chronic hepatitis C infection. N Engl J Med 364: 2429–2438, 2011 [DOI] [PubMed] [Google Scholar]
- 89.Sawinski D, Kaur N, Ajeti A, Trofe-Clark J, Lim M, Bleicher M, Goral S, Forde KA, Bloom RD: Successful treatment of Hepatitis C in renal transplant recipients with direct-acting antiviral agents [published online ahead of print November 25, 2015]. Am J Transplant doi: 10.1111/ajt.13620 [DOI] [PubMed] [Google Scholar]
- 90.Kamar N, Marion O, Rostaing L, Cointault O, Ribes D, Lavayssière L, Esposito L, Del Bello A, Métivier S, Barange K, Izopet J, Alric L: Efficacy and safety of sofosbuvir-based antiviral therapy to treat hepatitis C virus infection after kidney transplantation [published online ahead of print November 20, 2015]. Am J Transplant doi: 10.1111/ajt.13518 [DOI] [PubMed] [Google Scholar]
- 91.Pipili C, Cholongitas E: Pharmaceutical management of hepatitis B and C in liver and kidney transplant recipients. World J Gastrointest Pharmacol Ther 6: 105–110, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolffenbüttel L, Poli DD, Manfro RC, Gonçalves LF: Cyclosporine pharmacokinetics in anti-HCV+ patients. Clin Transplant 18: 654–660, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Oo YH, Dudley T, Nightingale P, Haydon G, Mutimer D: Tacrolimus and cyclosporin doses and blood levels in hepatitis C and alcoholic liver disease patients after liver transplantation. Liver Transpl 14: 81–87, 2008 [DOI] [PubMed] [Google Scholar]