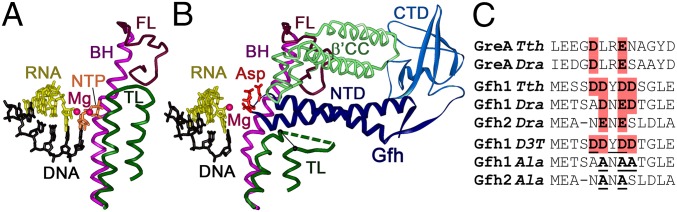
Fig. 1.
Interactions of Gfh factors with the RNAP active site. (A) Structure of the active TEC of Tth RNAP (Protein Data Bank accession number 1IW7) (10). The TL, BH, and F-loop (FL) are shown in green, magenta, and carmine, respectively. (B) Structure of Tth RNAP–Gfh1 complex (3AOI) (22). The acidic residues in the Gfh NTD tip are red; the β′ coiled-coil element (β′CC) interacting with Gfh C-terminal domain (CTD) at the entry of the secondary channel is light green. The position of the analyzed TL deletion (Δ1254–1272) is shown with a black line. (C) Alignment of the active sites of GreA and Gfh factors from Dra and Tth. Acidic residues in the NTD tip are shown in red; residues substituted in the mutant Gfh factors are boldfaced and underlined. The full GreA and Gfh sequences are shown in Fig. S1.