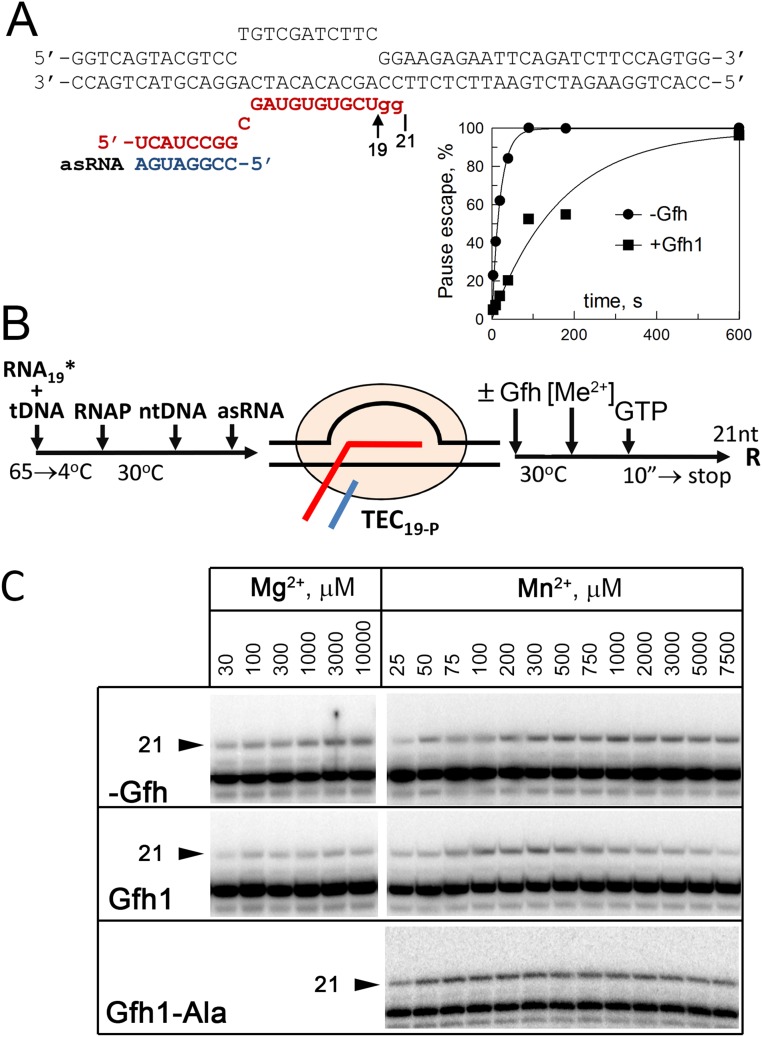
Fig. S7.
Analysis of Gfh effects on divalent metal ion binding. (A) Structure of the TEC used in the experiment (compare with Fig. S5B). The starting TEC contained 19-nt RNA corresponding to the site of pausing; the addition of GTP resulted in synthesis of 21-nt RNA transcript. The plot shows efficiencies of pause escape (normalized to the maximal RNA extension) by Dra RNAP in the TEC19-P measured in the absence and presence of Gfh1 (5 μM, 30 °C, 10 mM MnCl2). (B) Schematic of the experiment. The paused TEC19-P was incubated with Gfh (5 μM), and metal ions were added to various concentrations, followed by the addition of GTP (2 μM). The reaction was stopped after 10 s so that ≤25% of total RNA was extended. (C) Analysis of 21-nt RNA synthesis at different Me2+ concentrations in either the absence or presence of the Gfh1 factor and its mutant Gfh1-Ala variant. Note that absolute efficiencies of RNA extension in this experiment do not reflect any differences in the rates of RNA synthesis under corresponding conditions because of fluctuations in the efficiencies of TEC assembly in each particular reaction (because the reactions were performed in solution and not all RNA was bound by RNAP). To calculate the effects of Gfh on the apparent affinities of metal ions, the efficiencies of RNA extension at each Me2+ concentration were normalized to the maximal efficiency in the same titration experiment (Fig. 4B).