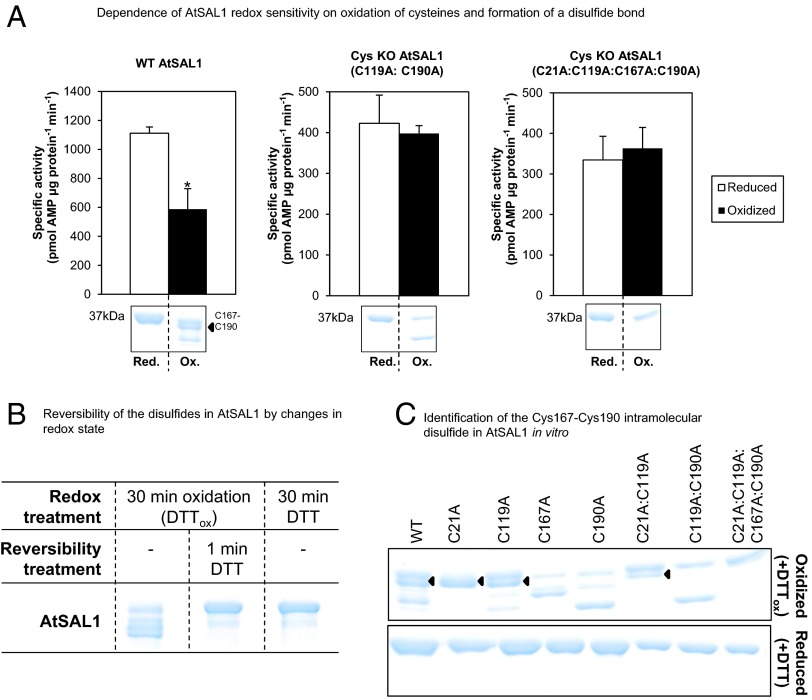
Fig. 2.
Regulation of AtSAL1 activity by redox state via its intramolecular Cys167–Cys190 disulfide. (A) Down-regulation of AtSAL1 activity by oxidation requires oxidation of cysteines because mutagenesis of cysteines to alanine in AtSAL1 abrogated redox sensitivity. The redox sensitivity correlates with a band directly beneath the full-length reduced protein (black arrow), which was determined to be a Cys167–Cys190 intramolecular disulfide (C). Vertical dashed lines indicate splicing and truncation of the gel shown in full in C. *P < 0.1. Activities of all proteins were assayed in the presence of 13.4 µM PAP. Means and SE of two independent experiments are shown. (B) Formation of disulfides in AtSAL1 by oxidation is rapidly reversed by returning the redox state to reducing conditions. Vertical dashed lines indicate splicing of the gel to show these three samples side by side; all samples were run on the same gel. (C) Determination of Cys–Cys disulfide pairs observed in WT AtSAL1 using cysteine to alanine substitution mutants of AtSAL1 under oxidation. Oxidized AtSAL1 proteins migrate at different rates to reduced AtSAL1 protein. The different Cys–Cys disulfide pairs were identified by cross-comparison with cysteine mutants: AtSAL1 containing a Cys167–Cys190 intramolecular disulfide (black triangles) migrates closest to reduced AtSAL1. The oxidized form is absent in all AtSAL1 mutants lacking either or both of Cys167 and Cys190. Other combinations such as the Cys21–Cys167 and Cys21–Cys190 disulfide did not correlate with the down-regulation of AtSAL1 activity by oxidation (A). These are likely nonspecifically formed during protein denaturation and SDS/PAGE. AtSAL1 containing the Cys167–Cys190 intramolecular disulfide is the only oxidized AtSAL1 species detected in endogenous plant protein samples pretreated with iodoacetamide to block reduced cysteines during protein extraction to prevent nonspecific disulfide formation (Fig. 6). Experiments were performed twice, with identical results.