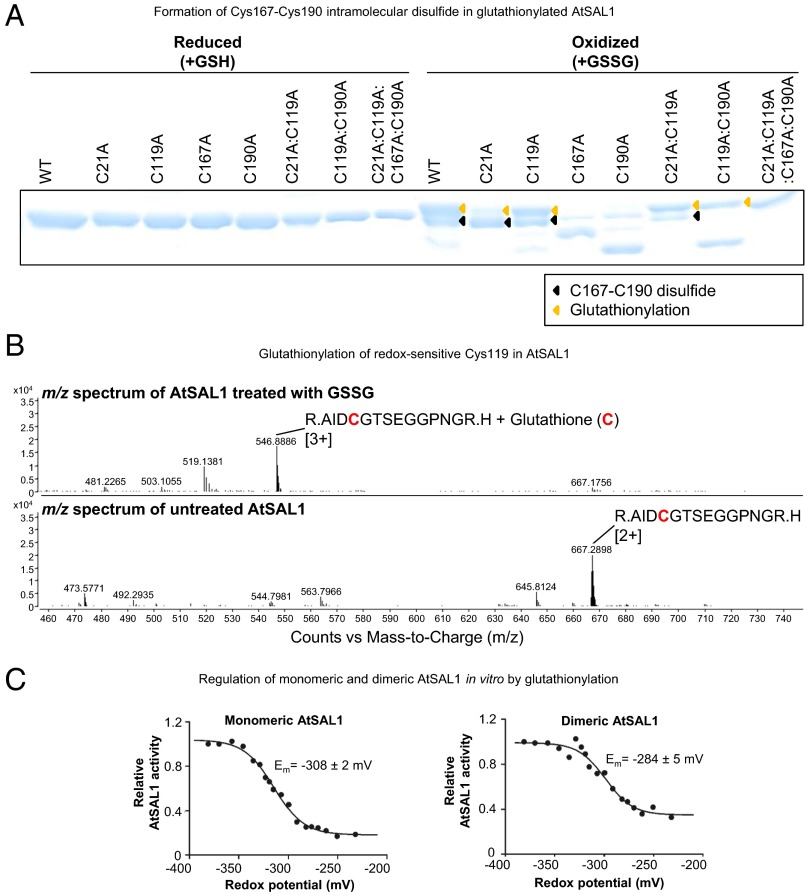
Fig. 5.
AtSAL1 can be regulated by glutathionylation at redox-sensitive cysteines. (A) Glutathionylation of AtSAL1 with oxidized glutathione (GSSG; yellow arrows) results in formation of the intramolecular C167–C190 disulfide (black arrows), presumably via the thiol–disulfide exchange mechanism (34). Identical results were obtained in two independent experiments. (B) Observed shift in mass consistent with cysteine glutathionylation in AtSAL1 treated with GSSG compared with untreated AtSAL1. A representative m/z spectrum for Cys119 is shown. Charge is indicated in brackets. (C) Both monomeric and dimeric AtSAL1 are sensitive to glutathionylation, with decrease in activity in redox titration with GSH/GSSG (a less negative potential is more oxidizing). The redox midpoint potential (Em) was close to physiological GSH/GSSG redox potential of Arabidopsis chloroplasts (35). Although dimeric AtSAL1 activity only decreased to 40% under fully oxidizing conditions compared with 10% for monomeric AtSAL1, the basal activity of dimeric AtSAL1 is already significantly lower than monomeric AtSAL1 under the same redox state (Table 1). Measurements were performed twice.