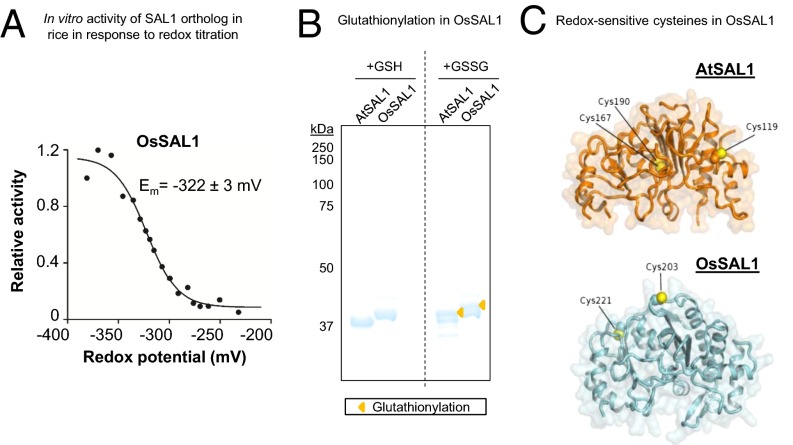
Fig. 7.
Biochemical and structural evidence for conservation of redox sensitivity in a rice SAL1 ortholog. (A) Redox titration on OsSAL1 shows that the protein is redox sensitive and has a redox midpoint potential (Em) in the physiologically relevant range. A less negative potential is more oxidizing. (B) Oxidation of AtSAL1 and OsSAL1 with GSSG similarly result in glutathionylation of the proteins, increasing their apparent molecular weight when resolved on nonreducing SDS/PAGE (yellow triangles). Vertical dashed lines indicate splicing and truncation of the gel to remove additional lanes not relevant to this result. (C) Comparison between redox-sensitive cysteine residues detected in structures of AtSAL1 and modeling of OsSAL1. Unlike AtSAL1 which contains both surface-exposed and intramolecular disulfide cysteines, OsSAL1 is predicted to contain surface exposed cysteines (marked in yellow). Both Cys203 and Cys221 of OsSAL1 are strongly conserved in Poaceae SAL1 orthologs (Fig. S5).