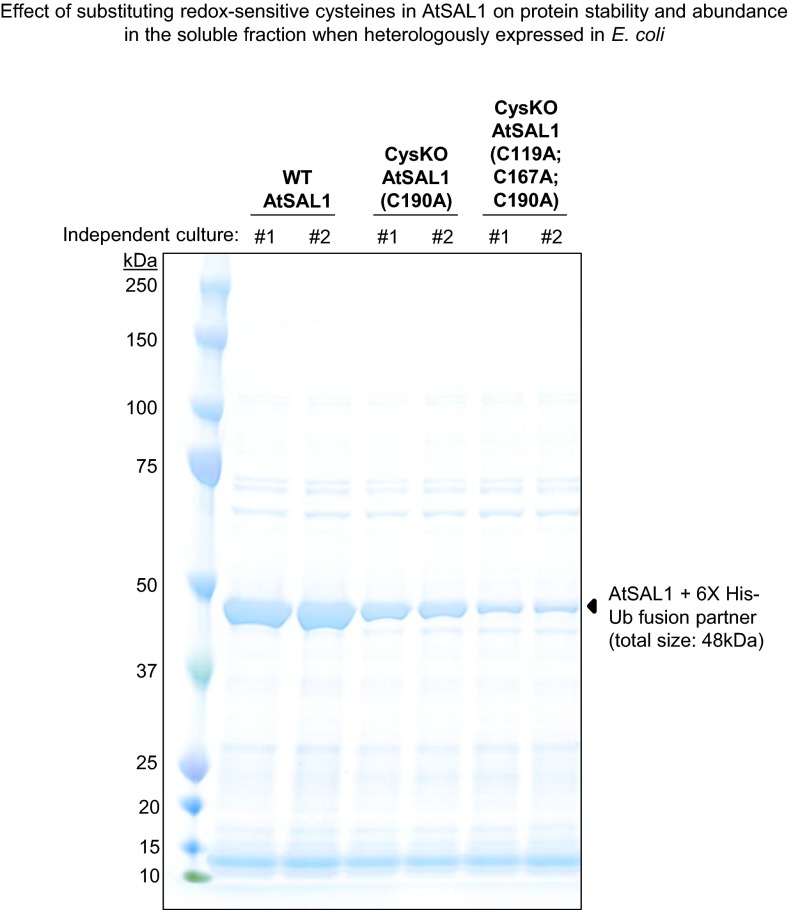
Fig. S4.
Cys–Ala mutations negatively affect AtSAL1 protein stability and abundance. The yield of soluble AtSAL1 protein is drastically decreased when the redox-sensitive Cys residues were mutagenized to Ala. The SDS/PAGE gel shows semipurified recombinant WT or mutated AtSAL1 proteins after a soluble protein fraction from 7 mL of induced E. coli cells was incubated with Ni-NTA beads in a 1.5 mL Eppendorf tube, washed with 20 mM imidazole, and the bound AtSAL1+6X His–Ub fusion proteins (black triangles) eluted with 250 mM imidazole. The abundance of soluble AtSAL1 protein decreased with increasing number of Cys–Ala substitutions. The negative effect of the Cys–Ala mutations was reproducible in two independent transformed bacterial colonies.