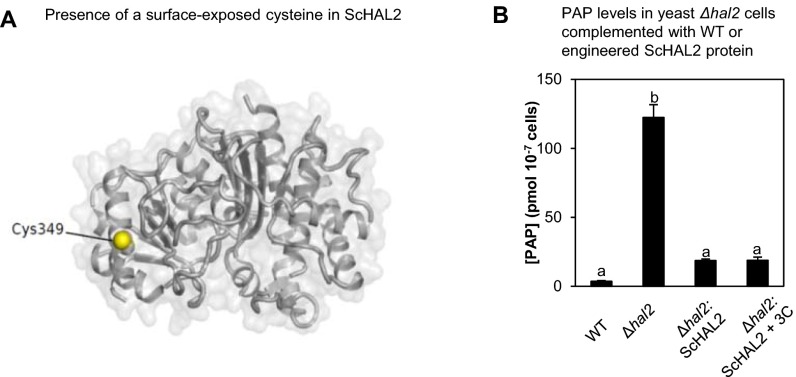
Fig. S6.
WT and engineered ScHAL2. (A) Presence of a surface-exposed Cys349 in the ScHAL2 crystal structure (1KA1), which may explain the redox-sensitive activity observed in Fig. 8B. (B) The WT ScHAL2 and ScHAL2+3C proteins are equally active in vivo when expressed in yeast cells deficient in ScHAL2 (Δhal2) because both proteins complement PAP levels in Δhal2 to similar levels, albeit still about 10-fold higher than WT. Similar results were obtained in two independent experiments.