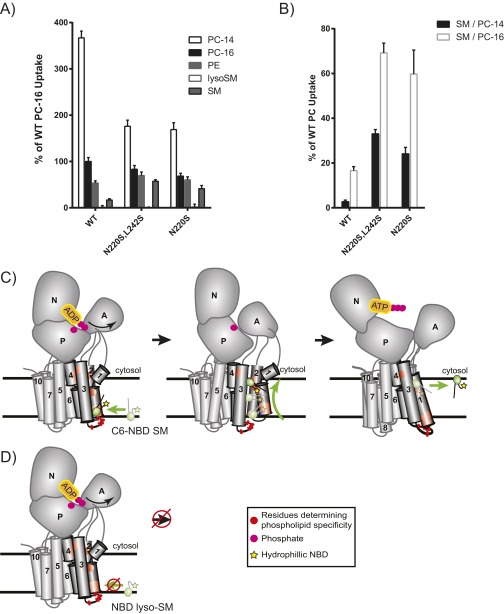
Fig. S3.
N220S substrate preference is not attributed to differences in substrate solubility or the length of the sn1 acyl position and long-chain base, and is unable to transport NBD-lyso-SM. When measuring SM selection and preference, we were concerned that our observations might be influenced by differences in substrate solubility in NBD-PC and NBD-SM. Differences in solubility would be predicted to alter substrate administration, incorporation into the plasma membrane, and ultimately accessibility to the P4-ATPase. Additionally, the sphingosine backbone carries a trans double bond at position 4. This trans double bond could alter the positioning of the long-chain base within the substrate-binding pocket of the enzyme, thereby influencing the ability to discriminate the length of this base. We measured the uptake of two different NBD-PC substrates with sn1 acyl-chain lengths of 14 and 16 carbons, respectively. Dnf1WT was found to take up PC-14 better than PC-16, as was consistent with its increased solubility. We also tested whether the location of the NBD label on the SM substrate was critical to its transport. Previous work has shown that the yeast Dnf1 enzyme is preferentially a lyso-PL transporter, suggesting that the Dnf1 enzyme recognizes the sn2 C6-NBD GPLs as it would lyso-PLs (38). We examined a lyso-SM substrate with an NBD label at the distal end of the sphingosine chain and found that this substrate could not be recognized by the SM+ mutants. These results likely suggest that the hydrophilic NBD group snorkels up to the aqueous interface of the membrane and perturbs lateral recognition of the lyso-SM substrate by Dnf1. (A) We found that Dnf1N220S,L242S and Dnf1N220S did not enhance the selection of a more soluble NBD-PC containing a shorter sn1 acyl chain but instead reduced the selection of PC-14 similar to that of PC-16. Additionally, the SM+ mutants were unable to recognize NBD-lyso-SM with a fluorophore attached to the distal end of the sphingosine chain. (B) Dnf1N220S,L242S and Dnf1N220S also increased the preference for NBD-SM when normalized to PC-14 and PC-16, suggesting that differences in discriminating substrate solubility or the length of the sn1/long-chain base acyl chain were not responsible for the observed increase in NBD-SM uptake. n ≥ 6 ± SEM. (C) We predict that the C-6 NBD PLs, like the natural substrates, are recognized laterally at the luminal/exofacial aspect of the P4-ATPase between TMs 1, 3, and 4. The PL then is transported through the enzyme and membrane bilayer as a function of ATP catalysis and is released from the P4-ATPase exit gate at the cytofacial aspect of the membrane. (D) Conversely, lyso-SM with the hydrophilic NBD label on the distal end of the long-chain base is predicted to cause the hydrophobic tail to snorkel up to the aqueous interface of the membrane (59), thereby preventing lateral recognition of the substrate at the entry gate.