Abstract
Cavernoma is a vascular hamartoma, which represents 10–20% of all central nervous system vascular malformations. The majority (80%) of them are supratentorial, while involvement of the cranial nerves and the optic pathways is extremely rare. The main clinical presentation of optochiasmatic cavernomas consists of chiasmatic apoplexy, which is a neurosurgical emergency. Here, we report a case in which the finding was incidentally detected in a 49-year-old man. We describe the imaging characteristics of the lesion in such a rare location, highlighting the role of magnetic resonance imaging (MRI) (specifically 3 Tesla) in the management of asymptomatic patients.
Keywords: Optochiasmatic, cavernoma, incidental
Introduction
The incidental detection of an optochiasmatic cavernoma (OCM) can raise problems for differential diagnosis and treatment, since there is a lack of data in the literature about asymptomatic patients. Generally, the malformation presents with visual impairment although some other clinical pictures are described, including subarachnoid haemorrhage. Here, we describe the main characteristics of this very unusual lesion found in an asymptomatic patient. We also underline relevant imaging features to reach a diagnosis, highlighting the role of magnetic resonance imaging (MRI) examinations performed with 3 Tesla equipment. We also suggest some clues for differential diagnosis.
Case report
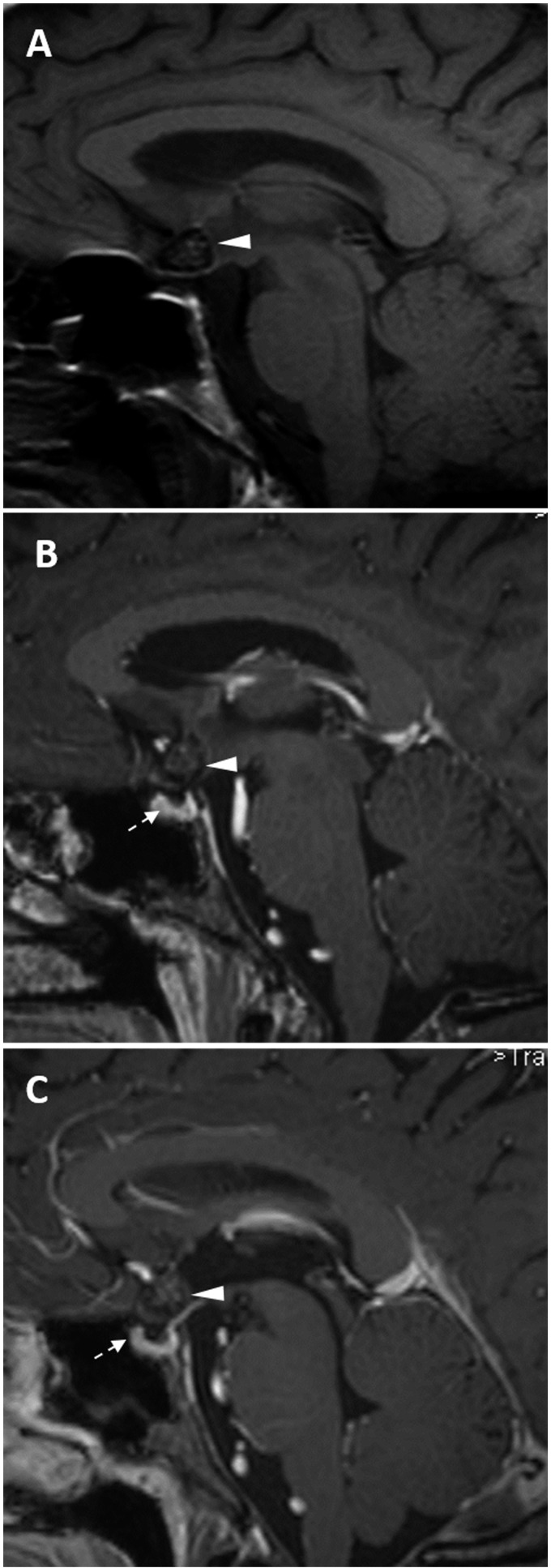
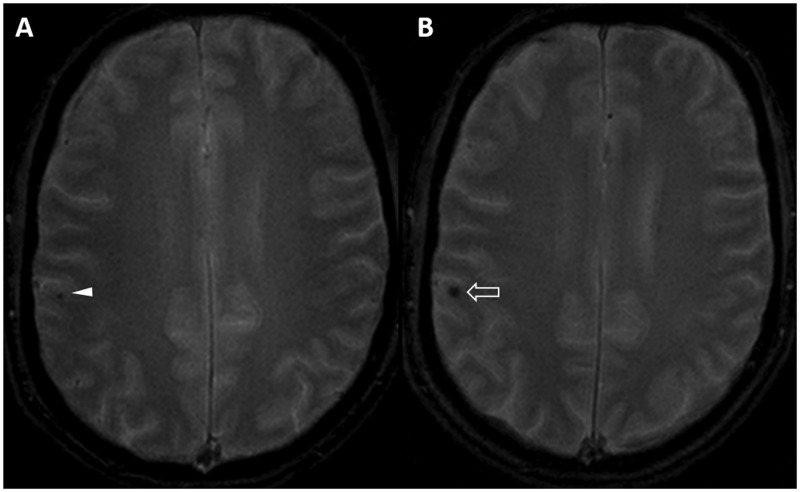
A 49-year-old man was referred to our institution for a second opinion after being studied at another hospital for dizziness. His history was negative for relevant pathologies, tinnitus, hearing loss and visual disturbances. Neurological and campimetric examinations were normal. A computed tomography (CT) examination had shown a hyper-dense rounded nodule at the suprasellar region. The finding was evocative of various pathologies (e.g. suprasellar aneurysm), but a subsequent MRI scan was not conclusive, since it suggested a possible craniopharyngioma. At our institute, the high contrast and anatomical resolution obtained with a 3 Tesla scanner allowed us to confirm the presence of a 1 cm lobulated lesion at the optochiasmatic junction with extension towards the left optic tract. The nodule presented with smooth, thin and hypo-intense borders, with an inhomogeneously hyper-intense core on the T2W images (Figures 1 and 2), and it did not enhance on T1W post-gadolinium images (Figures 3 and 4). The pituitary gland was normal, whereas the pituitary stalk appeared slightly displaced (Figures 1 and 3). In addition, on the T2* gradient recalled echo (GRE) images, an hypo-intense ‘blooming’ spot compatible with a ‘black dot-like’ vascular malformation was also found at the right post-Rolandic region (Figure 5). No alterations of the seventh/eighth cranial nerves and of the inner ear were found on either side. After the MRI, the hypothesis of an OCM was confirmed. Six months after diagnosis, the patient remains asymptomatic with normal campimetry, and a follow-up MRI did not show substantial changes to the chiasmatic lesion (Figure 3), whereas the black dot-like supratentorial alteration appeared slightly increased (Figure 5).
Figure 1.
T2 weighted turbo spin echo images (on three planes) show an inhomogeneously hyper-intense nodule 1 cm in size (arrow, a) with the so-called ‘popcorn’ appearance. The lesion is suprasellar, with slight displacement of the pituitary stalk (arrowhead, b).
Figure 2.
The T2 GRE images define the hemosiderin component of the lesion (arrow, ‘blooming effect’), making this sequence the ‘gold standard’ for detecting the malformation.
Figure 3.
T1 turbo spin echo images before (a) and after gadolinium injection (b, first MRI; c, follow-up MRI): the chiasmatic lesion (arrowheads) shows no enhancement and no enlargement at the follow-up MRI performed six months later. The hypophysis appears normal (dotted arrow, b–c).
Figure 4.
Axial (a) and coronal (b) T1 turbo spin echo post-contrastographic images. The chiasmatic involvement is evident (arrowheads on a: left optic nerve and optic tract; arrow on b: the chiasm).
Figure 5.
The T2 GRE images demonstrate a right post-Rolandic ‘black dot-like’ lesion consistent with a small vascular malformation. On follow-up, the alteration (empty arrow, b) appears slightly enlarged compared with the previous MRI realised by the same 3 Tesla scanner (arrowhead, a).
Discussion
We use the terms ‘cavernoma’ and ‘cavernous malformation’ (CM) to designate a vascular hamartoma1–3 with a prevalence of 0.4–0.9%4 within the general population. The malformation grows as a result of recurrent internal haemorrhages,5 unlike [hem]angiomas (the term also frequently reported in the literature) which are ‘true’ benign vascular neoplasms.2,6–8 CMs represent 10–20% of all central nervous system (CNS) vascular malformations,4,9 being located everywhere inside the CNS3 (80% supratentorial,4 especially subcortical in the frontal, temporal lobes);5,10–12 both sporadic (75%) and multiple/familial forms (10–30%) are recognised.2,13 CMs rarely involve the cranial nerves3–5,14 or the optic pathways (≤1%),1 and are typically 5–30 mm in size, consisting of aberrant, immature, thrombosed, low-flow vessels surrounded by a gliotic plan anchored to the adjacent nervous tissue11,14 (without neural tissue inside the nodule,1,3,10,11 unlike arteriovenous malformations5). The annual rate of bleeding increases from 0.25% to 20%,4 with a re-bleeding rate of 3.8–22.9%.1 Mass effect, oedema, irritation and ischemia induced on the surrounding tissue by intrinsic/extrinsic haemorrhages cause the clinical onset.4 Even if there is a lack of clear evidence,11,15 it is believed that the bleeding of OCMs induces symptoms more frequently than cavernomas located elsewhere.4,15,16 For this reason, symptomatic OCMs are considered a neurosurgical emergency.4,12,13 While seizures are the most common presentation of intracerebral cavernomas,11,12 the symptoms of OCMs (second to fourth decades1,12) are represented by a spectrum, with the most common picture consisting of chiasmatic apoplexy17 (acute chiasmal compression syndrome11: sudden frontal/retro-orbital headache, acute mono-/bilateral hypovisus, hemianopia). Subacute visual deterioration has been reported,1,3 especially in the weeks before acute onset. Subarachnoid haemorrhage1,3,5,10,11,13–15 and hypothalamic-hypophyseal disorders have also been reported (e.g. growth hormone/adrenocorticotropic hormone deficits, polyuria, polydipsia11,18). Symptoms are precipitated by alcohol consumption, pregnancy or physical effort.5,14
In our case, none of the above-described symptoms were found, since dizziness was not correlated with the chiasmatic lesion. Therefore, imaging acquired a pivotal role to detect and characterise the lesion to confirm its intrachiasmatic location, allowing the differentiation from more aggressive processes. At angiography, these lesions remain occult in most cases1,4,14 because of their low blood flow, internal thrombosis5 and absence of ectatic feeding vessels,1,5 even if a slight contrast blush can occasionally be found during the capillary phase.5,12 On CT scans, the OCMs appear as lobulated, well-marginated, slightly hyper-dense suprasellar nodules with poor/absent enhancement, associated with chiasmatic enlargement.1,5,14 Microcalcifications can be found in 40–60%,1,5,6,14 while fat components are absent. The CT morphologic features are also demonstrated by MRI, which is the most sensible and specific diagnostic tool.1,4,12,14 Because of the difficult and narrow space in which the OCMs are located, the high spatial/contrast resolution guaranteed by MRI, especially by 3 Tesla scanners, is crucial for diagnosis.4,15,16 The MRI signal behaviour of cavernomas has been classified by Zabramski.19 The typical ‘popcorn’ appearance on T2 weighted images consists of a thin, hypo-intense hemosiderin rim surrounding an inhomogeneous core of haemoglobin in various phases of degradation.2,3,5,14 It is important to note that the hypo-intense rim can lack in OCMs because of the lavage of the cerebrospinal fluid all around the lesion.1,14 A mono-/bilateral thickening of the chiasm represents another useful imaging clue.1,14 The T2*GRE4 and the susceptibility weighted images (SWI), in particular on 3 Tesla scanners, are essential to characterise and to increase the conspicuity of the malformation because of the hypo-intense ‘blooming’ effect of hemosiderin.6,16 Diffusion and perfusion weighted imaging are less useful for diagnosis (mask effect of hemosiderin). Extrinsic haemorrhages spreading inside the chiasm can mask the underlying lesion: in such cases, MRI follow-up can allow the correct diagnosis.5 At follow-up, OCMs can increase in volume (recurrent microbleedings), showing changes in their signal intensity.4,14
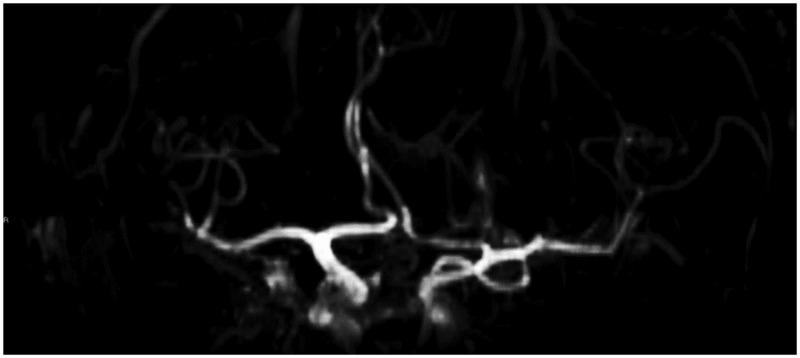
Our patient was asymptomatic. Thus, he did not undergo surgery, and histological confirmation was therefore lacking. In any case, the location of the lesion and its distinctive ‘popcorn’ appearance were indicative of the final diagnosis. Moreover, the further post-Rolandic ‘black dot-like’ vascular malformation that was detected supported the hypothesis, since an association of more than one cavernoma has been described.1,2,10,11,13 We had to exclude some other sellar/suprasellar lesions to reach the diagnosis. We ruled out an aneurysm (by magnetic resonance angiography, which showed a normal anatomy of the circle of Willis (Figure 6)), a craniopharyngioma (CRF) and a haemorrhagic pituitary adenoma, since these latter induce morphologic alterations of the pituitary gland and of the sella turcica. Furthermore, at MRI, both CRF and haemorrhagic adenoma can show a cystic appearance. In particular, CRFs present with hyper-intense cysts on T1 weighted images with enhancing solid components.1 Other sellar/suprasellar lesions to consider in differential diagnosis are summarised in Table 1.1,9,14,20
Figure 6.
MR-angiography image does not demonstrate abnormalities of the circle of Willis. In particular, no aneurysms of the anterior communicating artery were found.
Table 1.
Elements of differential diagnosis on MRI for some sellar/suprasellar lesions.
| Suprasellar aneurysm | • Even if partially thrombosed, flow void signal can be found • Communication with circle of Willis detectable at MRA |
| Craniopharyngioma | • Proteinaceous fluid-filled cysts hyper-intense on T1 weighted images • Enhancement of solid components • Pituitary/sellar morphologic alterations • Calcifications, hypo-intense on MRI, frequently associated |
| Haemorrhagic pituitary adenomas | • Partially cystic appearance with fluid-fluid levels • Sellar morphologic alterations • Pituitary gland not well-delineated |
| Arteriovenous malformation | • Nidus of ectatic vessels (‘bag of worms’, flow voids and contrast enhancement at MRI) |
| Optic/hypotalamic gliomas | • Haemorrhage/calcifications not typical • Only 10% in patients older than 20 years of age |
| Parasellar/optic sheath meningiomas | • Isointense with grey matter in all sequences • Strong enhancement • Optic nerve sheath involved, not optic chiasm • Sellar bone remodelling can be found • Dural tail |
| Germ-cell tumour, metastases (history of cancer, e.g. breast/lung cancer) | • Enhancement present |
| Dermoid/epidermoid | • Fat-like MRI appearance • Restriction on diffusion weighted imaging |
MRI, magnetic resonance imaging; MRA, magnetic resonance angiography.
Surgical excision is mandatory for symptomatic OCMs4,5: free margins of resection prevent re-haemorrhage,1,5,10,14 providing a good prognosis (80–90% clinical stabilisation and improvement/recovery of the visual function).1,3,11,13,16 Radiosurgery should be excluded to avoid re-bleeding and damage to the optic pathways.1,11,15 For asymptomatic OCMs, even if preventive surgery is considered,9,14,21 the best management could consist in follow-up22 (see comments in Deshmukh et al.13).
Conclusions
A discussion about the therapeutic work-up of incidental OCMs lacks evidence-based confirmation. As the reported case demonstrates, when surgery is not performed, the MRI is pivotal in the management of this condition, since in a ‘wait-and-see’ approach, this imaging modality represents the gold standard for follow-up.16 In asymptomatic patients, awareness of the correct diagnosis made by MRI could allow prompt surgery in case of future onset of symptoms, increasing the chances of visual recovery. The finding of more than one malformation could suggest an hereditary form of the condition. MRI screening of the patient’s family members could therefore be evaluated. Finally, we highlight that the conspicuity of OCMs is increased by using a 3 Tesla MRI scanner, which furthermore guarantees a higher anatomic/contrast resolution, which is useful to delineate the morphologic and signal intensity features of intrachiasmatic lesions better (Table 2).
Table 2.
Take home messages: role of MRI in asymptomatic patients.
| • Gold standard in differential diagnosis/follow-up | |
| • High grade of suspicion can allow prompt surgery and better prognosis when the lesion becomes symptomatic | |
| • Guide for neurosurgical approach | |
| • Detection of other vascular malformations throughout the central nervous system | |
| • Screening in patient’s family members if more than one lesion found | |
| • 3 Tesla scanners allow higher contrast/spatial resolution and increase the blooming effect and the lesion conspicuity | |
Funding
This work received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Liu JK, Lu Y, Raslan AM, et al. Cavernous malformations of the optic pathway and hypothalamus: analysis of 65 cases in the literature. Neurosurg Focus 2010; 29: E17. [DOI] [PubMed] [Google Scholar]
- 2.Osborn GA, Salzman KL, Jhaveri MD, et al. Diagnostic imaging: brain. Salt Lake City, UT: Amirsys, 2004, I-5-24; I-5-25; II-4–72.
- 3.Iwai Y, Yamanaka K, Nakajima H, et al. Cavernous angioma of the optic chiasm – case report. Neurol Med Chir (Tokyo) 1999; 39: 617–620. [DOI] [PubMed] [Google Scholar]
- 4.Rotondo M, Natale M, D’Avanzo R, et al. Cavernous malformations isolated from cranial nerves: unexpected diagnosis? Clin Neurol Neurosurg 2014; 126: 162–168. [DOI] [PubMed] [Google Scholar]
- 5.Arrue P, Thorn-Kany M, Vally P, et al. Cavernous hemangioma of the intracranial optic pathways: CT and MRI. J Comp Assist Tomogr 1999; 23: 357–361. [DOI] [PubMed] [Google Scholar]
- 6.Klostranec JM, Krings T. Neuroimaging of cerebral cavernous malformations. J Neurosurg Sci 2015; 59: 221–235. [PubMed] [Google Scholar]
- 7.Kashlan ON, Sack JA, Ramnath S. Cavernous angioma of the dural convexity mimicking a meningioma. Austin Neurosurg Open Access 2014; 1: 1019. [Google Scholar]
- 8.Vilanova JC, Barcelo J, Smirniotopoulos JG, et al. Hemangioma from head to toe: MR imaging with pathologic correlation. Radiographics 2004; 24: 367–385. [DOI] [PubMed] [Google Scholar]
- 9.Bourekas EC, Tzalonikou M, Christoforidis GA. Case 1. Cavernous hemangioma of the optic chiasm. AJR Am J Roentgenol 2000; 175: 888; 891. [DOI] [PubMed] [Google Scholar]
- 10.Alafaci C, Grasso G, Granata F, et al. Cavernous malformation of the optic chiasm: an uncommon location. Surg Neurol Int 2015; 6: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crocker M, Desouza R, King A, et al. Cavernous hemangioma of the optic chiasm: a surgical review. Skull Base 2008; 18: 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muta D, Nishi T, Koga K, et al. Cavernous malformation of the optic chiasm: case report. Br J Neurosurg 2006; 20: 312–315. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh VR, Albuquerque FC, Zabramski JM, et al. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery 2003; 53: 352–357. discussion 357. [DOI] [PubMed] [Google Scholar]
- 14.Ozer E, Kalemci O, Yucesoy K, et al. Optochiasmatic cavernous angioma: unexpected diagnosis. Case report. Neurol Med Chir (Tokyo) 2007; 47: 128–131. [DOI] [PubMed] [Google Scholar]
- 15.Panczykowski D, Piedra MP, Cetas JS, et al. Optochiasmatic cavernous hemangioma. Br J Neurosurg 2010; 24: 301–302. [DOI] [PubMed] [Google Scholar]
- 16.Lehner M, Fellner FA, Wurm G. Cavernous haemangiomas of the anterior visual pathways. Short review on occasion of an exceptional case. Acta Neurochir (Wien) 2006; 148: 571–578. discussion 578. [DOI] [PubMed] [Google Scholar]
- 17.Maitland CG, Abiko S, Hoyt WF, et al. Chiasmal apoplexy. Report of four cases. J Neurosurg 1982; 56: 118–122. [DOI] [PubMed] [Google Scholar]
- 18.Shkarubo AN, Serova NK, Tropinskaia OF, et al. [Chiasmatic cavernoma]. Zh Vopr Neirokhir Im N N Burdenko 2005; 20–1; discussion 21–22. [PubMed]
- 19.Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 1994; 80: 422–432. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Forell W. Intracranial pathology of the visual pathway. Eur J Radiol 2004; 49: 143–178. [DOI] [PubMed] [Google Scholar]
- 21.Elmaci I, Ates G, Kurtkaya O, et al. Chiasmal cavernous malformation. A rare cause of acute visual loss. J Neurosurg Sci 2000; 44: 226–229. [PubMed] [Google Scholar]
- 22.Newman H, Nevo M, Constantini S, et al. Chiasmal cavernoma: a rare cause of acute visual loss improved by prompt surgery. Pediatr Neurosurg 2008; 44: 414–417. [DOI] [PubMed] [Google Scholar]