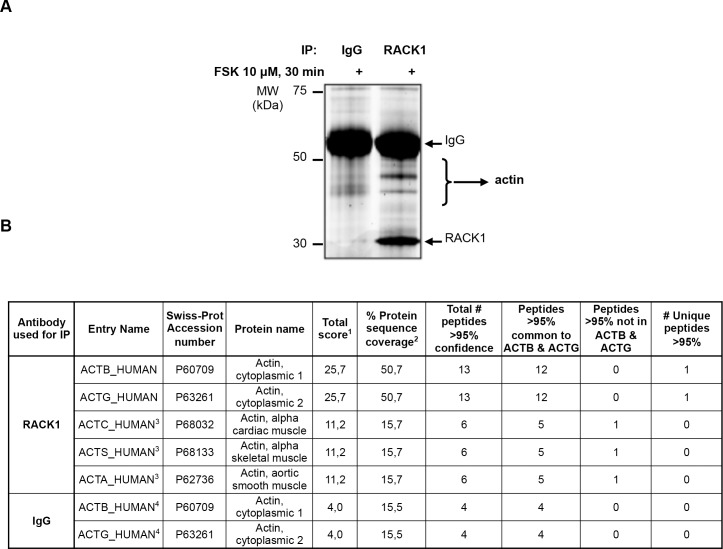
Fig 1. Mass spectrometry identification of actin as a binding partner of RACK1.
A SH-SY5Y cells were treated with 10 μM FSK for 30 min, RACK1 was IPed from cell lysate and proteins were resolved by SDS-PAGE which was stained with Deep Purple™ to visualize proteins. Gel slices from both RACK1 and control IgG IPs were digested in-gel and the resulting peptides were submitted to tandem mass spectrometry (MS/MS) sequencing for protein identification, n = 2. B Identification of actin as a putative RACK1 binding protein. β-actin (ACTB), γ-actin (ACTG), alpha skeletal muscle actin (ACTS), aortic smooth muscle actin (ACTA), alpha cardiac muscle actin (ACTC) were identified in the RACK1 IP. A sum of score of peptides that were matched to the best hit within the family of homologous protein sequences (1). Calculation of the percent sequence coverage included all peptides matched to the protein, i.e., both unique and common peptides (2). Differentiation between ACTC, ACTS and ACTA proteins was not possible on the basis of the data since the identified peptides were common to all of them (3). Differentiation between β-actin and γ-actin proteins was not possible on the basis of the data since the identified peptides were common to both isoforms (4).