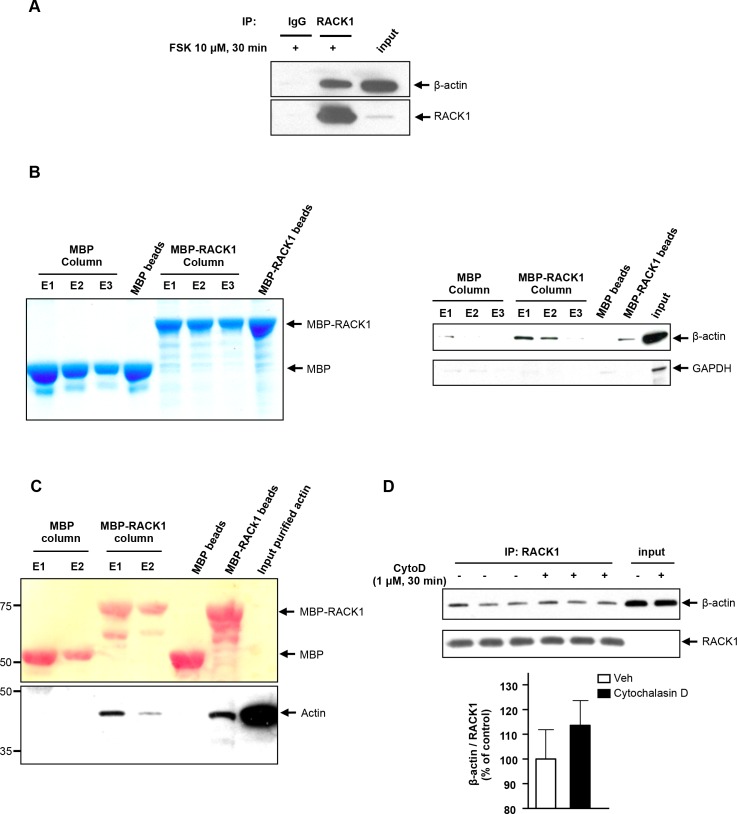
Fig 2. β-actin is a direct binding partner of RACK1.
A SH-SY5Y cells were treated with 10 μM FSK for 30 min, and were then lysed in IP buffer. RACK1 was IPed from whole cell lysate and the co-IPed proteins were resolved by SDS-PAGE. Endogenous RACK1 and β-actin were analyzed by western blot analysis. B Recombinant MBP and MBP-RACK1 were immobilized on an amylose resin column and incubated with SH-SY5Y lysate previously treated with 10 μM FSK for 30 min. After washing, bound proteins were eluted 3 times with 50 mM maltose (E1, E2 and E3) or with loading buffer (MBP and MBP-RACK1 beads). Proteins were resolved by SDS-PAGE and the presence of β-actin and GAPDH was determined by western blot (right panel). The amount of MBP and MBP-RACK1 recovered after elution was also controlled with colloidal coomassie blue staining (left panel). n = 2. C The experiment was conducted as in panel B except that immobilized recombinant MBP and MBP-RACK1 were incubated with pure non-muscle actin. After washing, proteins were eluted twice with 50 mM maltose (E1 and E2) or with loading buffer (bead lanes). Eluted proteins were resolved by SDS-PAGE, transferred to nitrocellulose membrane and stained with Ponceau S to control the amount of MBP and MBP-RACK1 (upper panel). The presence of actin was subsequently determined by western blot analysis using a pan actin antibody (lower panel). D SH-SY5Y cells were incubated with vehicle or 1 μM cytochalasin D (cytoD) for 30 min, and then lysed in IP buffer. RACK1 was immunoprecipitated from whole cell lysate and proteins were resolved by SDS-PAGE. RACK1 and β-actin were revealed by western blot analysis. Histogram depicts the mean ratio of β-actin to RACK1, expressed as percent control ± SEM. Two-tailed unpaired t-test, p = 0.40. A n = 3, B and C n = 2, D n = 6.