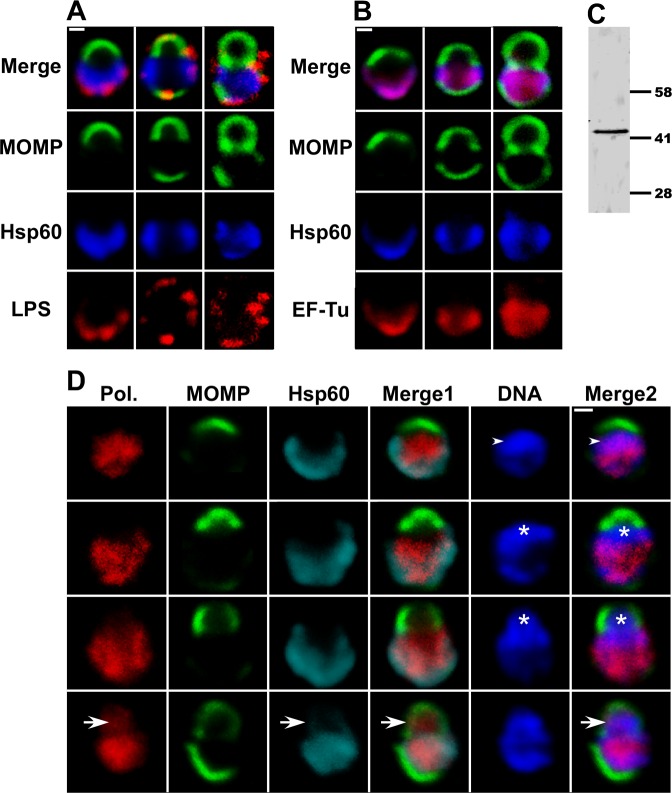
Fig 6. Localization of cytosolic and membrane markers during the polarized cell division process of C. trachomatis.
(A, B, and D) HeLa cells were infected with C. trachomatis serovar L2 and fixed at 11 hours post-infection. The cells were then permeabilized and LPS localization was determined by incubating cells with anti-LPS mouse monoclonal antibodies (A; red), RNA polymerase β subunit localization was determined using mouse monoclonal antibodies (Pol. in D; red), EF-Tu localization was determined using anti-EF-Tu mouse polyclonal antibodies (B; red). The distribution of the mouse primary antibodies was visualized by incubating cells with donkey anti-mouse IgG conjugated to Alexa Fluor 568. Hsp60 localization was visualized using anti-Hsp60 rabbit polyclonal antibodies followed by donkey anti-rabbit IgG conjugated to Alexa Fluor 633 (A and B—blue; D—cyan) and MOMP localization was visualized using anti-MOMP goat polyclonal antibodies followed by donkey anti-goat IgG conjugated to Alexa Fluor 488 (A, B, and D; green). In some instances, the cells were stained with Hoechst (D; blue) prior to confocal analysis. Each panel contains various intermediates in the polarized cell division process. Arrowheads in D denote regions in a nascent daughter cell where the DNA and the RNA polymerase β subunit co-localize. Asterisks in D mark nascent daughter cells that contain DNA but lack the RNA polymerase β subunit and Hsp60. Arrows in D point to region where the RNA polymerase β subunit and the bacterial chromosome co-localize in a nascent daughter cell that does not yet contain Hsp60. In Panel D, Merge1: merge of MOMP/ RNA polymerase β subunit/Hsp60; Merge2: merge of MOMP/ RNA polymerase β subunit/DNA. White bars in A, B, and D are 0.5μ. (C) A lysate prepared from HeLa cells infected with C. trachomatis at 24 hours post-infection was subjected to immunoblotting analysis with the EF-Tu antibodies. A single 43kDa species that matches the predicted molecular mass of EF-Tu was observed.