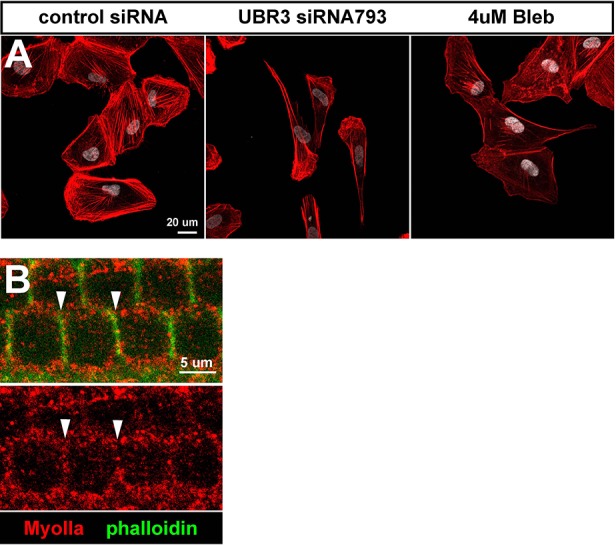
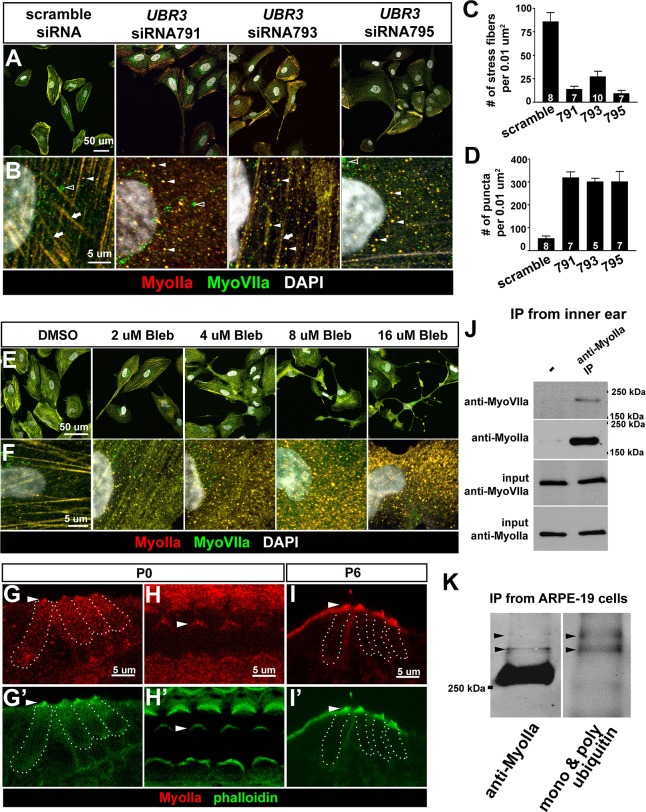
Figure 5. The function of Ubr3 is conserved in vertebrate cells.
(A–B) Cultured ARPE-19 human cells transfected with indicated siRNAs are co-immunolabeled by anti-MyoIIa and anti-MyoVIIa antibodies and DAPI (white). Arrows: stress fibers. Arrowheads: MyoIIa-MyoVIIa co-localized puncta. Empty arrowheads: MyoVIIa positive, MyoIIa negative puncta. (C–D) Quantifications of stress fiber number and MyoIIa-MyoVIIa puncta shown in A–B. Error bars show SEM. Numbers of cells quantified are shown in the columns. (E, F) ARPE-19 cells treated with indicated concentrations of blebbistatin for 30 min followed by immunolabeling with anti-MyoIIa (red) and anti-MyoVIIa (green) antibodies and DAPI. Low concentration (2–4 μM) of blebbistatin treatment resulted in elongated ARPE-19 cells with protrusions, similar as UBR3 siRNA-treated cells. Further increasing the dosage of blebbistatin (8–16 μM) resulted in cells with a more branched, tree-like morphology. The number of puncta correlated with the concentration of blebbistatin, suggesting a specific change in MyoII-MyoVIIa interactions. (G–G’) Immunolabeling of a cochlear section from a neonatal mouse with anti-MyoIIa (red) and phalloidin (green). Hair cells are outlined by dashed lines. (H–H’) Surface view of whole mount cochlea from a neonatal mouse immunolabeled with anti-MyoIIa (red) and phalloidin (green). Arrowheads mark V-shaped stereocilia (labeled by phalloidin, green). (I–I’) Immunolabeling of cochlear section from P6 pup with anti-MyoIIa (red) and phalloidin (green). Arrowheads mark stereocilia (shown by phalloidin staining in green). (J) Co-immunoprecipitation with anti-MyoIIa antibody from P5 cochlear lysate followed by western blotting. (K) MyoIIa was purified from ARPE-19 cells through immuno-precipitation, followed by western blot. Arrowheads indicate ubiquitinated MyoIIa (shown by FK2 antibody).
Figure 5—figure supplement 1. MyoII proteins localize close to cell membrane in the hair cells of mouse cochlea.