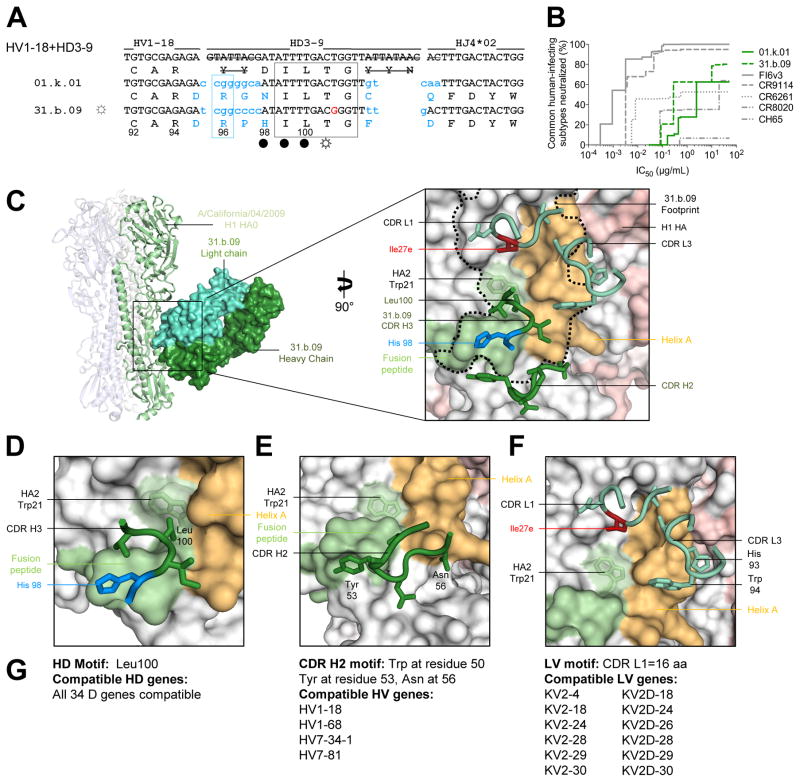
Figure 3. A multidonor HV1-18+HD3-9 class of broadly neutralizing antibodies.
(A) Immunoglobulin heavy chains utilizing germline genes HV1-18, HD3-9 and HJ4, with sequences annotated as described in Figure 2A. (B) Neutralization breadth-potency curve for HV1-18+HD 3-9 antibodies on a panel of influenza A viruses that includes subtypes known to infect humans. (C) Co-crystal structure of Fab 31.b.09 in complex with an H1 trimer (A/California/04/2009). Fab heavy and light chains colored dark green and light green respectively and depicted in surface representation, while the H1 HA is depicted in ribbon and colored blue, green, and white. Inset: Interacting CDR loops of the 31.b.09 Fab are shown in ribbon and sticks and colored as in panel (A) with the antibody footprint outlined. (D) A conserved motif within the CDR H3 inserts into the highly conserved Trp21 pocket of HA while also interacting with the fusion peptide. (E) The HV1-18 encoded CDR H2 also interacts with the opposing side of the fusion peptide. (F) Light chain interactions from CDR L3 and CDR L1 also contribute to the antibody binding surface area. (G) Analysis of antibody gene compatibility. See also Figures S3–S7 and Tables S3–S7.