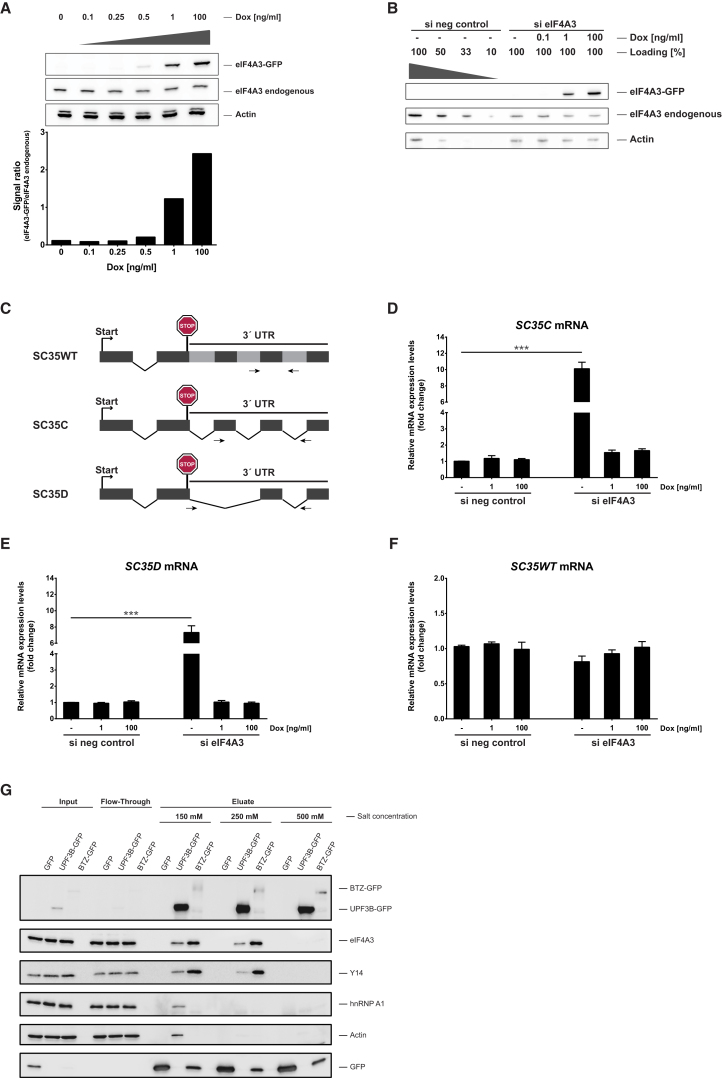
Figure 1.
The Experimental HeLa Cell System Expressing Fully Functional EJC-GFP Fusion Proteins Is Suitable for iCLIP Experiments
(A) Titration of doxycycline demonstrated a concentration-dependent increase of the eIF4A3-GFP fusion proteins and that a concentration of 1 ng/ml doxycycline was best suited to achieve an expression close to the endogenous level. This image shows a representative immunoblot of three biologically independent experiments. Endogenous and recombinant eIF4A3 were stained concurrently with an α-eIF4A3 antibody.
(B) Representative immunoblot after siRNA treatment of three biologically independent experiments is shown. The endogenous and recombinant eIF4A3 were stained concurrently with an α-eIF4A3 antibody.
(C) Schematic drawing of three SC35 mRNA isoforms adapted from Sureau et al. (2001). The grey boxes in SC35WT mRNA represent RNA regions that are spliced out in the other isoforms. The arrows show the position of the primers that were used for the amplification of the transcripts (Table S7).
(D and E) Upregulation of SC35C (D) and SC35D (E) transcripts after depletion of endogenous eIF4A3 and rescue of efficient NMD upon induction of the fusion protein with doxycycline.
(F) The expression of the NMD-insensitive SC35WT isoform did not change under the different conditions. The error bars represent SEM, and p values were calculated by one-way ANOVA with Dunnett's multiple comparison test (∗∗∗p value < 0.001 with n = 3–5 independent biological experiments).
(G) CoIPs show that the EJC core proteins eIF4A3 and Y14 were stably associated with UPF3B and BTZ under salt concentrations of 150 and 250 mM NaCl and disassembled at 500 mM NaCl. Therefore, 500 mM NaCl was used for the subsequent iCLIP experiments. GFP, BTZ-GFP, and UPF3B-GFP were stained concurrently with an α-GFP antibody.