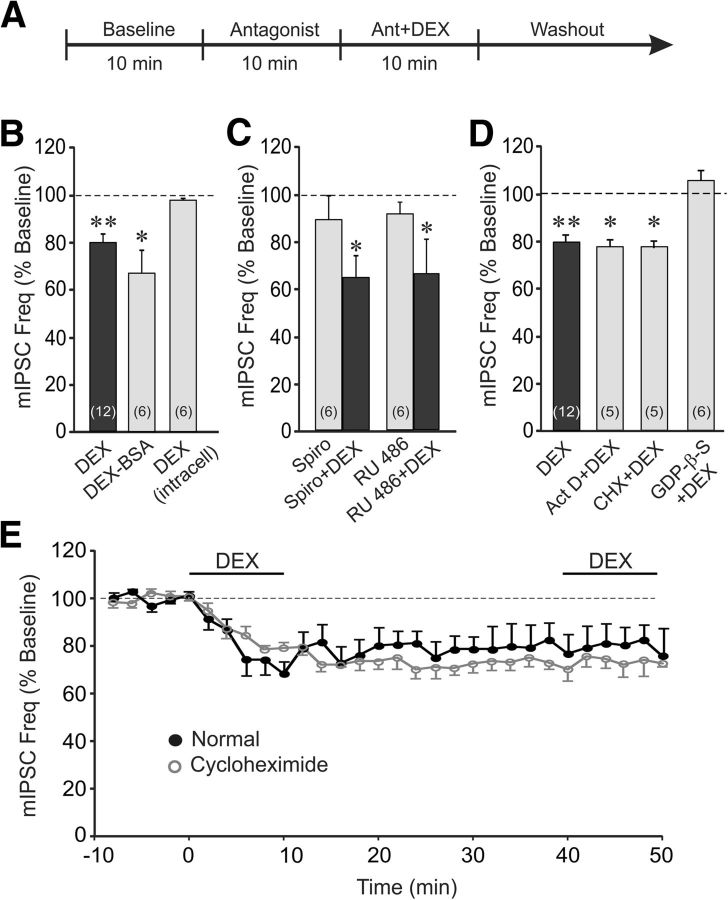
Figure 2.
The glucocorticoid effect was mediated by a membrane-initiated, nongenomic, G-protein-dependent signaling mechanism. A, The timeline of the whole-cell recording paradigm used for data presented in C and D, except that the antagonists in D were applied intracellularly via the patch pipette throughout the recordings. The timeline used for data presented in B was the same as that illustrated in Figure 1A. B, Bath application of the membrane-impermeant DEX-BSA conjugate maintained the steroid's suppressive effect on mIPSC frequency, whereas direct intracellular application of DEX via the patch pipette had no effect on mIPSC frequency. C, The DEX suppression of mIPSC frequency was not blocked by prior application of antagonists of the nuclear mineralocorticoid receptor spironolactone (Spiro+DEX) or the nuclear glucocorticoid receptor RU 486 (RU 486+DEX). The antagonists alone were without effect on the relative mIPSC frequency (Spiro and RU 486). D, Blockade of gene transcription and protein synthesis with intracellular application of actinomycin D (Act D) and cycloheximide (CHX), respectively, via the patch pipette did not block the DEX suppression of mIPSC frequency. Blockade of postsynaptic G-protein activity with intracellular application of GDP-β-S via the patch pipette abolished the decrease in mIPSC frequency induced by DEX. E, Time course of mean relative changes in mIPSC frequency elicited by two successive applications of DEX in the absence and presence of the protein synthesis inhibitor cycloheximide in the bath. Inhibiting protein synthesis did not block the maintenance of the glucocorticoid response, nor did it block the occlusion of the response to a second glucocorticoid application. *p < 0.05; **p < 0.01.