Abstract
For decades, there has been enormous scientific interest in tropical savannahs and grasslands, fuelled by the recognition that they are a dynamic and potentially unstable biome, requiring periodic disturbance for their maintenance. However, that scientific interest has not translated into widespread appreciation of, and concern about threats to, their biodiversity. In terms of biodiversity, grassy biomes are considered poor cousins of the other dominant biome of the tropics—forests. Simple notions of grassy biomes being species-poor cannot be supported; for some key taxa, such as vascular plants, this may be valid, but for others it is not. Here, we use an analysis of existing data to demonstrate that high-rainfall tropical grassy biomes (TGBs) have vertebrate species richness comparable with that of forests, despite having lower plant diversity. The Neotropics stand out in terms of both overall vertebrate species richness and number of range-restricted vertebrate species in TGBs. Given high rates of land-cover conversion in Neotropical grassy biomes, they should be a high priority for conservation and greater inclusion in protected areas. Fire needs to be actively maintained in these systems, and in many cases re-introduced after decades of inappropriate fire exclusion. The relative intactness of TGBs in Africa and Australia make them the least vulnerable to biodiversity loss in the immediate future. We argue that, like forests, TGBs should be recognized as a critical—but increasingly threatened—store of global biodiversity.
This article is part of the themed issue ‘Tropical grassy biomes: linking ecology, human use and conservation’.
Keywords: biodiversity conservation, diversity, grassland, tropical forest, tropical savannah, rainforest
1. Introduction
The Earth's tropical landscapes are dominated by two strongly contrasting biomes: savannahs and grasslands on the one hand and closed-canopy forests on the other (figure 1a). Together they support much of the Earth's biodiversity, and both have been subject to similar high rates of land-cover conversion in recent decades. Somewhat paradoxically, however, savannahs and grasslands—henceforth, tropical grassy biomes (TGBs)—have remained conspicuously absent from the global discourse on land clearing and biodiversity loss. Only very recently has society begun to appreciate the biodiversity values of TGBs, and the extent to which they are under threat [6,7]. The historical underappreciation of the conservation value of TGBs has stemmed from a widespread and persistent misconception that they are anthropogenically ‘derived’, representing forests degraded by human activities [8]. Clearly, some TGBs have been derived from forest [9]. However, there is also a widespread and entrenched misunderstanding of the status of ancient TGBs that dominate the tropics, wherever disturbance or aridity severely limit woody cover [10,11]. Ancient TGBs have long evolutionary histories, as demonstrated by their high species diversity, endemism and functionally distinct biotas [12], including floras with many adaptations to frequent disturbance by fire and grazing [13]. TGBs are only just beginning to be recognized as globally important reservoirs of biodiversity.
Figure 1.
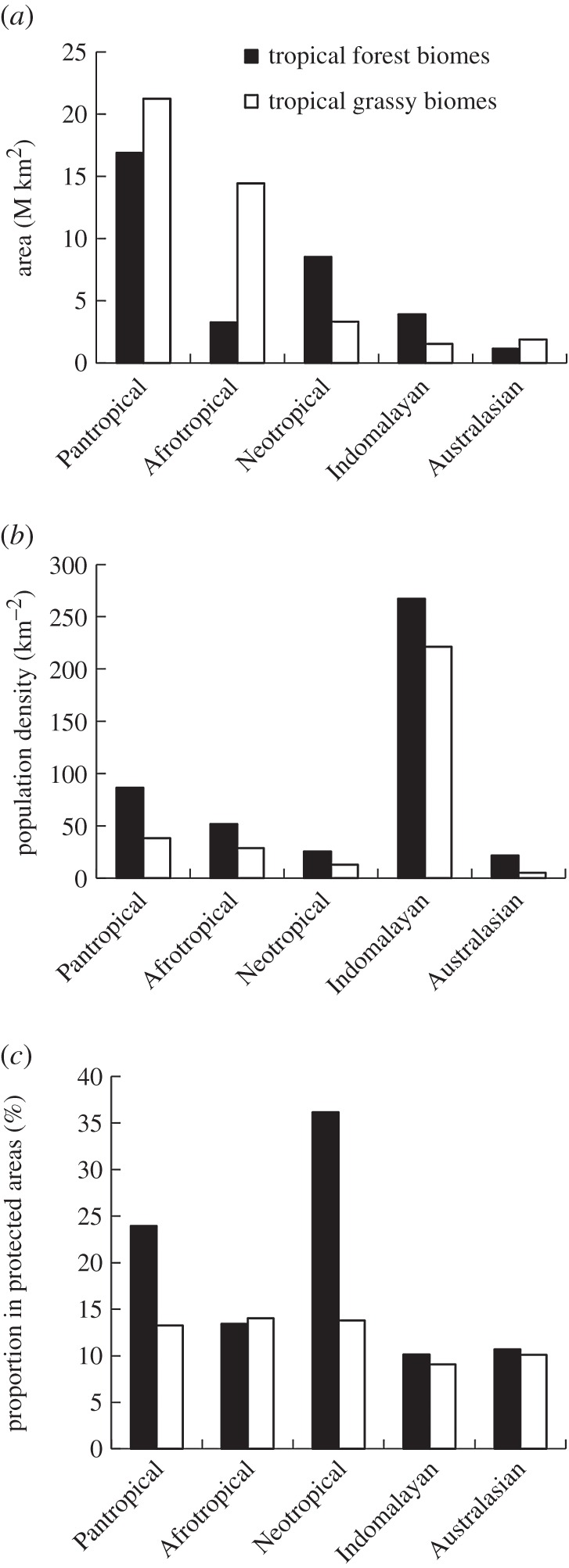
The (a) land area, (b) population density and (c) proportional inclusion in protected areas, of tropical forest and grassy biomes. These are shown separately for the entire tropics (Pantropical), and the four biogeographic realms [1] which dominate the tropics. Population density is based on [2]; protected areas are from [3]. Because of their limited area at a pantropical scale, the Nearctic and Oceanian realms were omitted. The realms are shown in decreasing order of total area in the tropics. In (a), mean population density for each ecoregion was derived from [2]. In (c), protected area data were obtained from the World Database on Protected Areas [4], following the methods of [5]. Protected area estimates include all IUCN Protected Area Management Categories (I–VI) as well as areas not designated with an IUCN category.
Tropical forests are renowned for their remarkable diversity of trees, yet diversity of other plant life forms can be very high in savannahs. For example, 230 (mostly herbaceous) vascular plant species have been recorded in a single 0.1 ha plot in the Brazilian Cerrado [14]. Moreover, diversity is much more conspicuous in TGBs than in tropical forests. The great diversity of grass-layer plants is there for all to see (even if only at certain times, such as following fire), rather than towering 30 m or more overhead. In forests, the vast majority of invertebrate species are either secreted in the litter layer or out of sight in the canopy, whereas the savannah invertebrate fauna is concentrated in the grass-layer or on open ground [15,16]. Most of the tropics' mammalian megafauna occur in open savannah rather than forest. Large vertebrates are highly visible in savannahs, but in forest are typically hidden by dense foliage and low light. The tropical savannah biome has particular significance for our own species, because it was the cradle of hominid evolution [6].
While we emphasize the need for conservation of TGBs in general, there are clear ecological and evolutionary differences among regions dominated by grassy biomes [17–19]. Just as major differences among tropical forest regions have been recognized [20,21], there is a need to consider how savannah regions differ too. There are some obvious differences in biotic composition due to biogeographic history. For example, the dominant trees of Australian savannahs, eucalypts, do not occur on other continents. Fungus-growing termites (family Macrotermitinae) are restricted to the Old World [20], and fungus-growing ants (tribe Attini) occur only in the Neotropics. Australian and Neotropical savannahs support contrasting ant faunas that are dominated by arid-adapted and forest-adapted elements, respectively, reflecting their contrasting biogeographic histories [21]. Such compositional differences can have important functional implications. For example, eucalypts have been suggested to be unique among savannah trees in their ability to escape the recruitment bottleneck imposed by high fire frequency [22,23]. Ants are major herbivores in Neotropical savannahs, as they collect substrate for their fungal gardens [24]. Neotropical savannahs have an extremely diverse fauna of tree-nesting ants, a habit which is very uncommon in savannahs elsewhere [21]. Intra-biome comparisons not only provide important insights into the ecology of these systems, but also help identify regionally distinct conservation priorities [20]. Given the divergent biogeographic histories of TGBs globally [25,26], combined with differing threats, it is likely that conservation needs and priorities will vary.
Despite the growing appreciation of TGBs and the threats they face, there remains a poor understanding of their biodiversity values at a global scale. Here, we seek to redress this by analysing global patterns of species richness of vertebrates and vascular plants. We build on recent regional-scale research to evaluate the biodiversity consequences of land-cover conversion in TGBs [27]. Specifically, we examine how species richness of TGBs compares with that of tropical forests in each of the tropical biogeographic realms. We also compare the extent to which TGBs and forests are formally protected, and how this varies regionally. We acknowledge the very high biodiversity values of savannah invertebrates (see box 1), but our analysis ignores invertebrate diversity due to limited data availability.
Box 1. Diversity of savannah ants.
Ants are the dominant faunal group in terms of biomass and energy flow in tropical forests, and such forests are widely regarded as the global centres of ant diversity. However, ant diversity can be similarly high in tropical savannahs, especially in Australia and the Neotropics [19,28]. For example, Australian savannahs pack up to 150 ant species per hectare, and such high diversity is maintained with increasing aridity down to at least 600 mm mean annual rainfall [28]. A remarkable 15 species from a single ant genus have been recorded in a single 10 × 10 m savannah plot [15]. Ant diversity in Australian savannahs is even more remarkable in that almost all species nest in the ground and forage on the soil surface, and therefore potentially interact with each other. This contrasts with tropical forests, where ant species show very strong vertical stratification, with separate litter-dwelling, epigaeic and arboreal communities that are largely independent of each other [16].
Ant diversity in Australian savannahs is strongly promoted by fire, which maintains the open habitat conditions to which the species are adapted. With increasing time since fire, there is a progressive decline in abundance of arid-adapted taxa, an increase in abundance of highly generalized, more shade-tolerant taxa, and an overall reduction in diversity [29]. Succession to forest sees the complete elimination of open savannah species, colonization by specialist forest taxa with Indomalayan affinities, and reduction of diversity to less than 50 species ha–1 [30].
2. A global analysis of species richness of tropical grassy biomes
In terms of their perceived biodiversity values, savannahs have been overshadowed by tropical forests. There can be no doubt that tropical forests contain some of the most species-rich plant and animal communities on the Earth [31]. For some groups, such as trees, tropical forest regions are unsurpassed in diversity [32]. However, for taxa associated with open biomes—such as grasses, megaherbivores (both grazers and browsers), and the large carnivores that prey on them—their centres of diversity lie in regions dominated by non-forest, grassy biomes [33]. These systems represent some of the most iconic and spectacular examples of complex terrestrial foodwebs—such as the Serengeti in East Africa [34]—and will inevitably feature prominently in humanity's efforts to conserve the natural world.
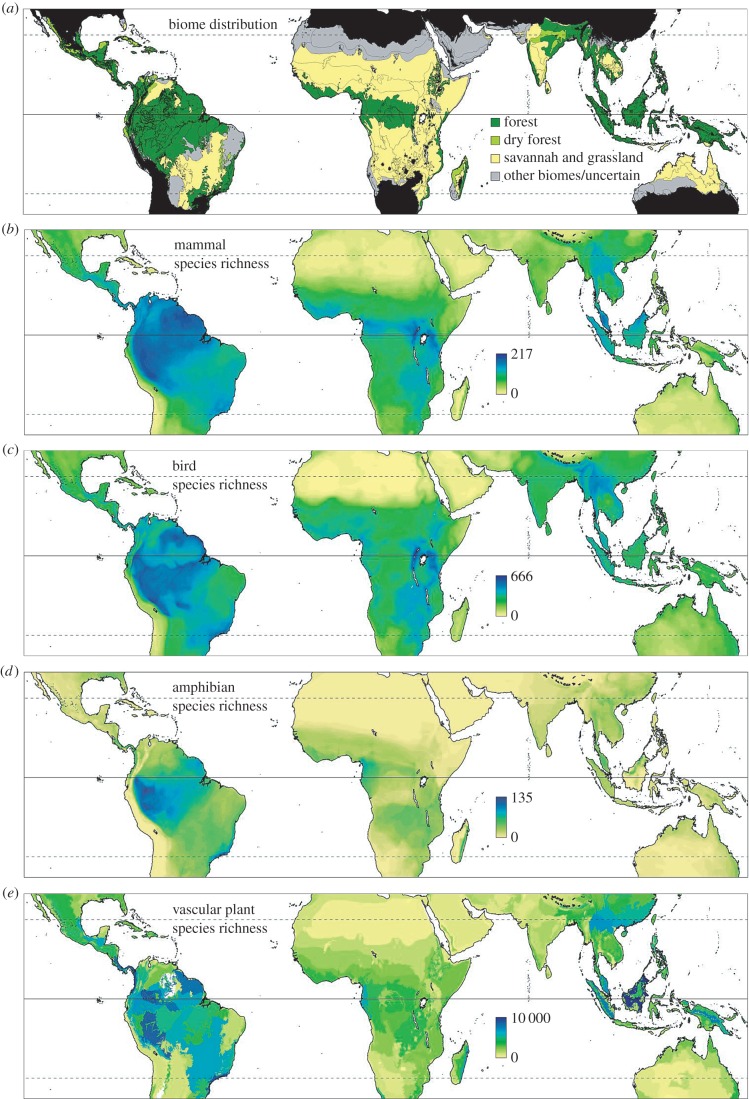
The recent availability of globally consistent maps of the species richness of key taxa has allowed significant advances in our understanding of the global distribution of biodiversity [5,35]. Using such data, there have been many analyses of the relationships between climate and species richness [36]. However, remarkably little attention has been paid to differences in biodiversity between biomes within the same climate zone. This is particularly important for the seasonal tropics, where forest and savannah can exist as alternative stable states [37–40]. Here, we use global datasets of species richness of three important vertebrate taxa (mammals, birds, amphibians: figure 2b,c) [5] and vascular plants (figure 2e) [35], to compare species richness of TGBs with that of tropical forest biomes, and to examine variation in species richness among different TGB regions.
Figure 2.
(a) The broad distribution of forest, dry forest and grassy biomes in tropical climate zones, defined here as having minimum monthly temperature greater than or equal to 15°C. Areas outside the tropical climate zones are shaded black. The biome map is generally based on the ‘ecoregions’ of Olson et al. [41]—with each ecoregion allocated a dominant biome (see the electronic supplementary material, appendix S1). Boundaries between ecoregions are indicated by fine black lines. Variation in the species richness of mammals, birds, amphibians and vascular plants throughout the land areas of the tropics are shown in panels (b–e). The vertebrate species richness data relate to total mean species richness for 10 × 10 km cells, and are from Jenkins et al. [5]. The plant data relate to species richness of each ecoregion, and are from Kier et al. [35]. In (e), there are two white patches in South America, where plant richness data were not available. The solid black line indicates the Equator and the dashed lines indicate the Tropic of Cancer and Tropic of Capricorn.
(a). Analytical methods
Our primary aim is to compare species richness between TGBs and tropical forest biomes. We used the ‘ecoregions’ map of Olson et al. [41] as the sampling unit in our analysis. We focused on those areas with a tropical climate, which we defined on the basis of temperature. Köppen [42] defined tropical climates as having monthly mean temperatures consistently above 18°C. However, we followed Murphy & Bowman [37] and used a cut-off of 15°C as this corresponds more closely to the geographical tropics (i.e. latitude less than or equal to 23.5°), and encompasses the Earth's major TGB regions. For each of the 825 ecoregions, we estimated monthly mean temperatures from the WorldClim dataset [43] (http://www.worldclim.org/), averaged across each ecoregion, and excluded ecoregions from the analysis if they had any month with mean temperature less than 15°C. We also excluded island ecoregions with area less than 100 000 km2 (slightly smaller than the island of Java), as we considered that small islands were likely to have relatively few species.
(i). Spatial datasets
We examined 10 response variables: nine related to vertebrate species richness and one related to vascular plant species richness. The vertebrate data were extracted from nine high-resolution global maps of local species richness (total number of species in 10 × 10 km cells): mammals (all, range-restricted, threatened), birds (all, range-restricted, threatened) and amphibians (all, range-restricted, threatened). The global maps of vertebrate species richness were from Jenkins et al. [5] (http://biodiversitymapping.org/). They were created by stacking digital range maps of individual species provided by the IUCN Red List (http://www.iucnredlist.org/), for mammals and amphibians, and Birdlife International (http://www.birdlife.org/datazone/), for birds. Range-restricted species were assumed to be those with a geographical range less than the median geographical range for that group of vertebrates. Threatened species were those listed as vulnerable, endangered or critically endangered on the IUCN Red List. For each ecoregion, the mean value of each vertebrate response variable was calculated.
The tenth response variable, the number of vascular plant species in each ecoregion, was obtained from Kier et al. [35]. These regional species richness estimates were based on one of four methods, depending on data quality: collation and interpretation of published data; the use of species–area curves to extrapolate richness; the use of taxon-based data, and estimates derived from other ecoregions within the same biome. Kier et al. [35] provided a range for each species richness estimate, so for the purposes of our analysis we assumed the midpoint of this range.
The original authors of the species richness datasets did not discuss sampling bias, but this is potentially an issue, with, for example, more-accessible and better-studied regions appearing to have higher species richness. We are unable to assess the extent to which this could potentially bias our evaluation of the most biodiverse ecoregions.
As explanatory variables, we used mean annual rainfall (averaged across each ecoregion), from the WorldClim dataset [43], the absolute value of latitude of the geographical centre of the ecoregion, and whether the ecoregion was predominantly grassy or forest. There is no globally accurate map of the TGBs, so we initially based our classifications on the dominant biome classes provided for each ecoregion by Olson et al. [41]. We classed ecoregions as: tropical forest if their biome type was ‘moist broadleaf forests’ or ‘coniferous forests’; TGB if their biome type was ‘grasslands, savannahs and shrublands’, ‘flooded grasslands and savannahs’ or ‘montane grasslands and shrublands’ (electronic supplementary material, table S1). We excluded ‘deserts and xeric shrublands’ as these typically have a discontinuous C4 grass layer.
All ecoregions were assessed to verify the classification of Olson et al. [41] and reclassified if necessary to tropical forest or TGB based on our knowledge of these ecoregions. The major changes were to class six coniferous forest ecoregions and 15 dry forest ecoregions as TGBs (electronic supplementary material, table S1), given that they are known to support a well-developed grass layer and are subject to frequent fire [44]. This almost certainly applies to the dry (dipterocarp) forests of mainland Southeast Asia, and most likely also to Indian dry forests [13]. Where we were uncertain about the status of dry forests as TGBs, particularly for Mesoamerica, we took a cautionary approach and excluded the ecoregions from our analysis. We acknowledge the uncertainty in some classifications but believe this approach is a more accurate representation of the Earth's TGBs.
Each ecoregion was grouped into one of six biogeographic realms [1]: Afrotropic, Neotropic, Indomalaya, Australasia, Oceania, Nearctic.
(ii). Statistical analysis
For each response variable, we compared eight candidate models using the Akaike Information Criterion (AICc):
response ∼ realm,
response ∼ realm × log(rainfall),
response ∼ realm × latitude,
response ∼ realm × [log(rainfall) + latitude],
response ∼ realm + TGB,
response ∼ realm × log(rainfall) + TGB,
response ∼ realm × latitude + TGB,
response ∼ realm × [log(rainfall) + latitude] + TGB.
The categorical variable ‘realm’ represented the biogeographic realms. There were only two ecoregions in the Nearctic realm, so these were grouped with Neotropical ecoregions. ‘Rainfall’ was mean annual rainfall. ‘Latitude’ was the absolute value of latitude. ‘TGB’ was a binary variable representing whether the ecoregion was tropical forest or a TGB. In the case of vascular plants, species richness was the total number of species in each ecoregion, which we expected to be positively correlated with the area of the ecoregion. Hence, we included a term representing the log of ecoregion area (km2) in each model, as an interaction with realm (electronic supplementary material, table S2d).
The models were fit as generalized least-squares regression models in R [45]. There was evidence of strong spatial autocorrelation of model residuals, so we specified a spatial autocorrelation structure in the models [46]. We compared three different autocorrelation structures (spherical, exponential, rational quadratic), and selected the one which minimized AICc. We considered it likely that the model variance would decrease with increasing area of the ecoregion, so we weighted the ecoregions according to their area using weighted generalized least squares.
(iii). Ranking ecoregions according to species richness
Within ecoregions dominated by TGBs, we sought to identify those with the highest species richness of (i) major vertebrate groups (mammals, birds, amphibians) and (ii) vascular plants. To derive a composite species richness score for vertebrates collectively, we standardized mammal, bird and amphibian species richness by dividing by the global mean for each group. We then calculated the mean of the three standardized scores.
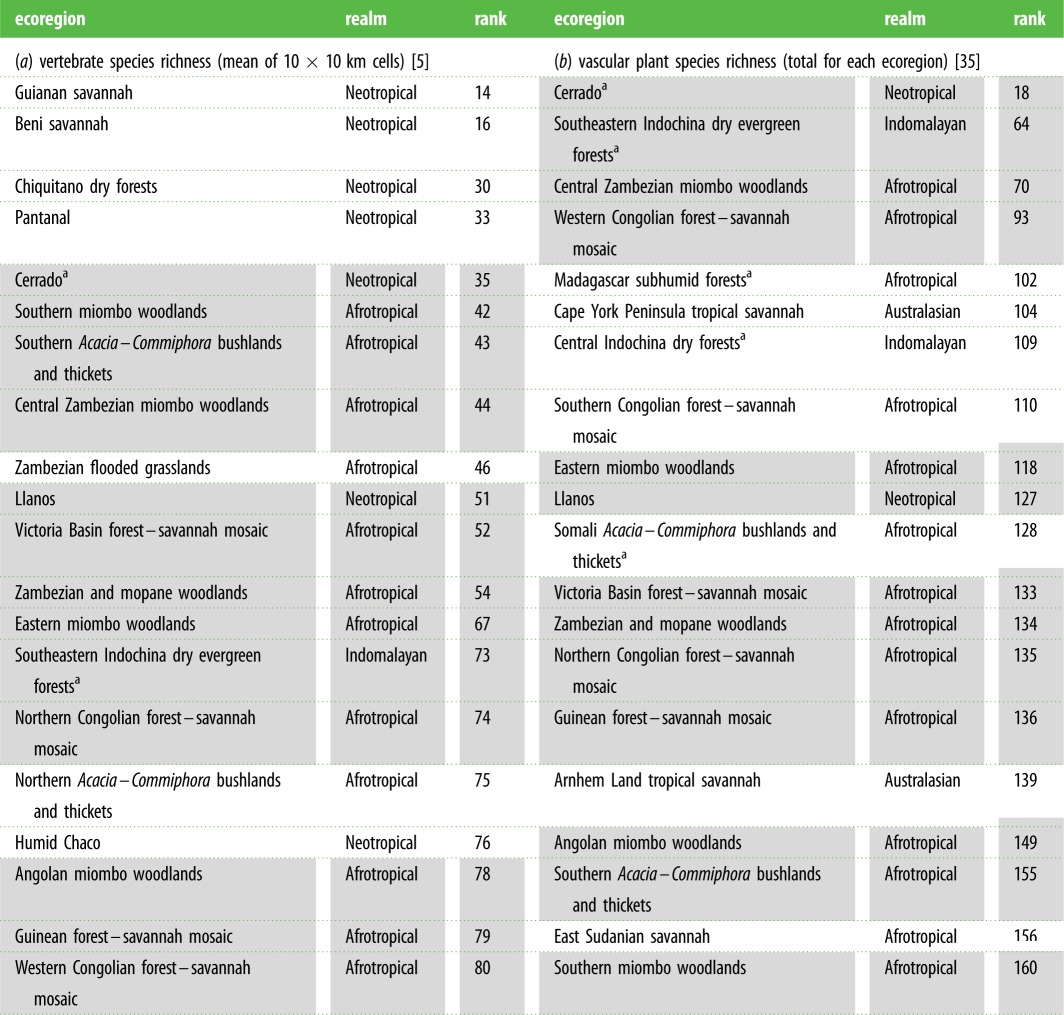
We then ranked the Earth's 825 ecoregions based on species richness (rank 1 = highest species richness; rank 825 = lowest species richness), firstly for vertebrate species richness (based on the composite score) and then for plant species richness (based on the total number of vascular plant species). The rankings for major TGB ecoregions (i.e. larger than the median ecoregion size, 62 300 km2) are reported in table 1.
Table 1.
The Earth's most species-rich tropical ecoregions dominated by grassy biomes (including only ecoregions larger than the median, 62 300 km2). Vertebrate species richness (a) relates to the mean of species richness in 10 × 10 km cells, while for vascular plants (b), it is the total number of species present in the ecoregion. The ‘rank’ represents the global ranking of each ecoregion (out of 412 large ecoregions) in terms of species richness; in the case of vertebrates, it is a composite ranking, taking into account the species richness of all three taxa. The ecoregions are sorted according to the rankings. The cell shading indicates ecoregions which are common between (a) and (b), i.e. they are in the 20 most highly ranked TGBs in terms of both vertebrate and vascular plant species richness.
 |
aEcoregions considered biodiversity hotspots by Myers et al. [47].
(b). Comparing tropical grassy biomes with tropical forests
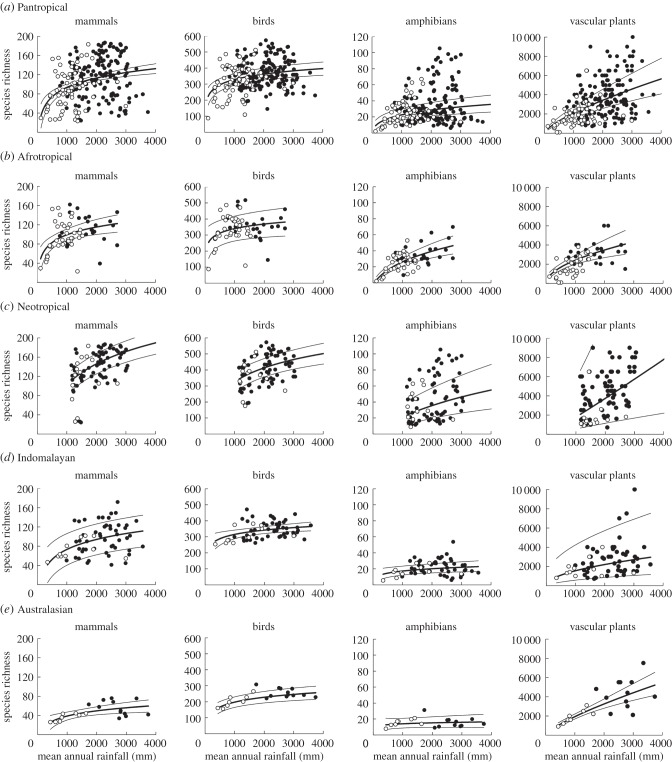
Our analysis suggests that mean species richness is consistently lower in TGBs than in forest biomes, in some cases (vascular plants and amphibians) very markedly so (figure 3). However, to some extent this can be attributed to lower rainfall than to biome type per se. The well-known tendency of TGBs to occur at lower rainfall [37,48] is clear in each of the major biogeographic realms of the tropics (figure 4). However, where tropical forest and TGBs co-occur along the rainfall gradient, there appears to be little difference in vertebrate species richness (figure 4).
Figure 3.
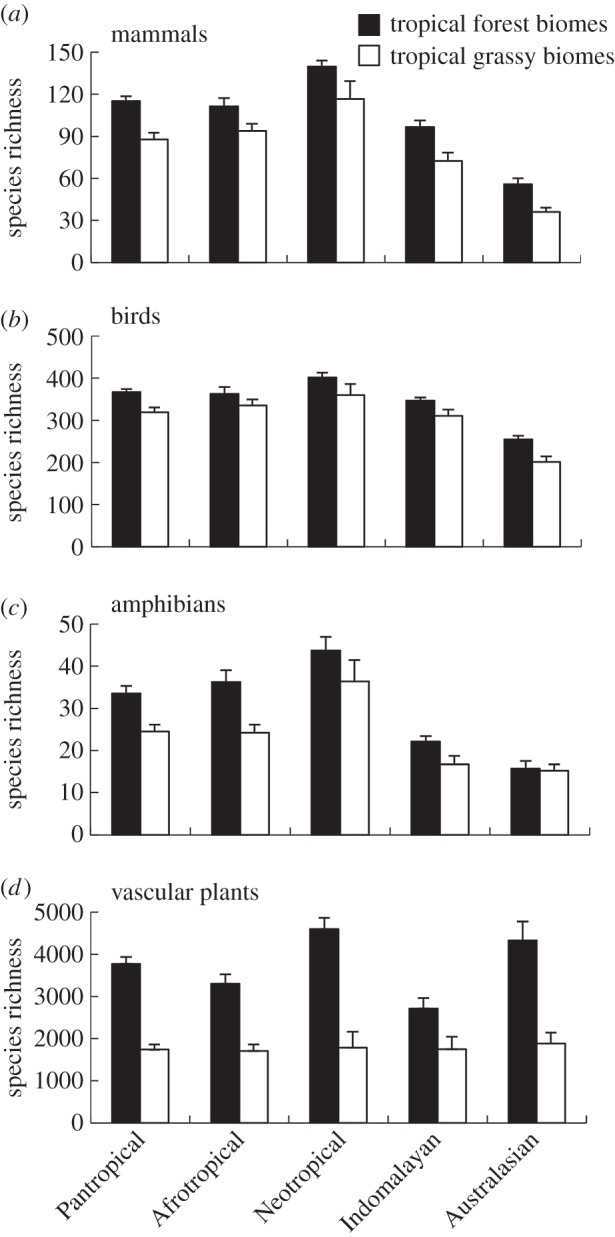
Comparison of species richness between tropical forest and TGBs in each biogeographic realm, for key vertebrate groups: (a) mammals, (b) birds and (c) amphibians, as well as (d) vascular plants. The means are calculated from the values for each tropical ecoregion. The error bars indicate standard error of the mean (of ecoregions).
Figure 4.
The relationship between mean species richness and mean annual rainfall, for key vertebrate groups (mammals, birds and amphibians) as well as vascular plants, shown for (a) the whole tropics (pantropical), and (b–e) separately for each tropical biogeographic realm. Each data point represents either the mean of 10 × 10 km cells (for vertebrates) or the total (for plants) species richness for an ecoregion. The open circles indicate TGBs, and the filled circles indicate forest biomes.
Indeed, spatially explicit generalized least-squares regression models—which account for the effects of biogeographic realm, rainfall and latitude—show little difference in vertebrate species richness between tropical forest and TGBs (figure 5a; electronic supplementary material, table S2). This finding is starkly at-odds with notions of TGBs being extremely species-poor relative to tropical forests. That said, species richness of vascular plants was markedly lower in TGBs; at median rainfall and latitude (1640 mm and 10.5°, respectively) an ecoregion dominated by TGBs could be expected to have over 40% fewer vascular plant species than a tropical forest ecoregion (figure 5a; electronic supplementary material, table S2).
Figure 5.
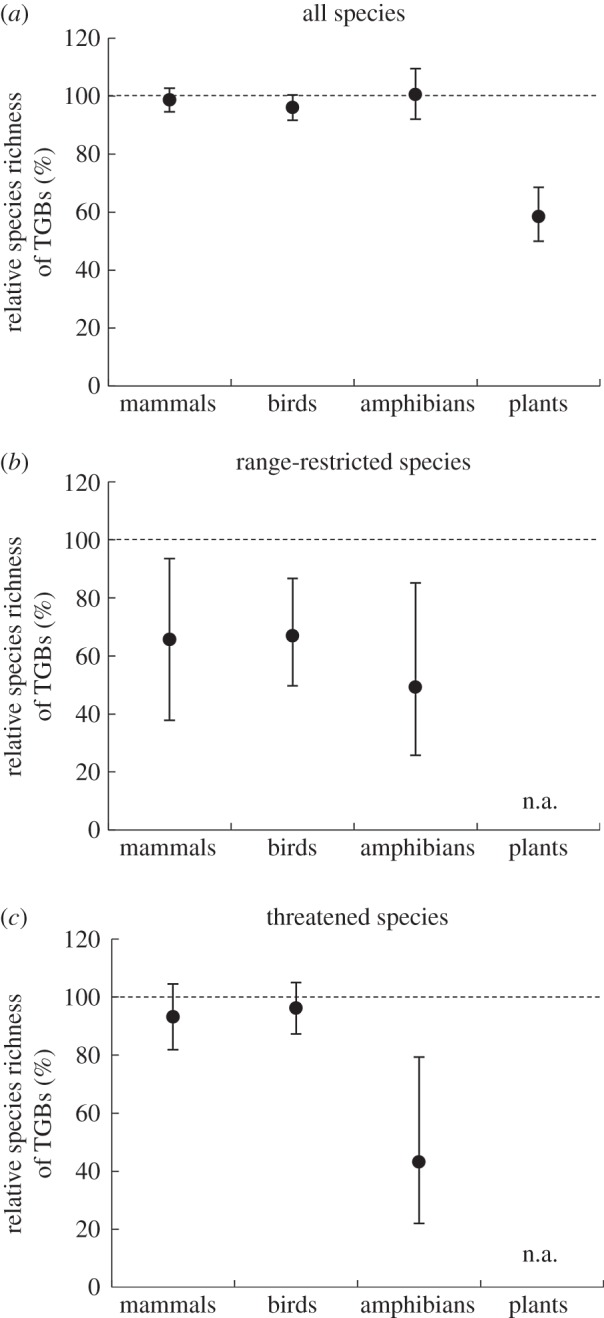
(a–c) Relative species richness of TGBs (expressed as a proportion of species richness of tropical forests), for key vertebrate groups (mammals, birds and amphibians) and vascular plants, accounting for differences in rainfall and latitude. The model predictions assume a mean annual rainfall of 1640 mm and latitude of 10.5° (the median of the tropical ecoregions in our dataset), using the global models from our analysis (vertebrate species richness ∼ realm × [log(rainfall) + latitude] + TGB; plant species richness ∼ log(area) × realm × [log(rainfall) + latitude] + TGB). The error bars indicate 95% confidence intervals of the predictions.
While overall vertebrate species richness did not differ markedly between tropical forest and TGBs, the richness of range-restricted species (an indicator of levels of endemism) were very markedly lower in TGBs (figure 5b). Species richness of threatened amphibians, but not threatened mammals or birds, was also markedly lower (figure 5c).
(c). Where are the most diverse tropical grassy biomes?
In terms of vertebrate species richness, the Neotropics and to a lesser extent the Afrotropics stand out clearly as having the most diverse TGBs (figure 3a–c and table 1a). Of the 20 TGB ecoregions with the highest mean species richness of vertebrates, only one is from outside the Neotropics or Afrotropics (‘Southeastern Indochina dry evergreen forests’ in Indomalaya; table 1a). TGB ecoregions in the Neotropics have the highest concentrations of ranged-restricted vertebrates (electronic supplementary material, figure S1), making them particularly important for biodiversity conservation.
While they have vertebrate species richness typical of high-rainfall tropical regions, the Indomalayan TGBs have particularly high concentrations of threatened birds and, to a lesser extent, mammals (electronic supplementary material, figure S1). This is most likely a product of high rates of historical land-cover conversion in India and mainland Southeast Asia, coupled with very high human population densities (and associated hunting pressure; figure 1b).
Species richness of vascular plants in TGBs was less variable across biogeographic realms (figure 3d). Of the 20 TGB ecoregions with the highest number of vascular plant species, there were at least two from each of the four major tropical realms (table 1b). TGB ecoregions which were among the most species rich in terms of both vertebrates and vascular plants included the Cerrado and Llanos of the Neotropics, a range of miombo- and mopane-dominated ecoregions of southern and central Africa, as well as dry tropical forests in Indochina.
Although the vascular plant dataset we used for our analysis [35] contains no information on the richness of different life forms, it is likely that the high species richness of tropical forests is contributed mainly by woody plants, particularly trees and lianas [32]. By contrast, TGBs are likely to have much higher species richness of grasses and forbs [14].
3. Threats to the biodiversity of tropical grassy biomes
Large-scale land-cover conversion is the most serious threat to TGB biodiversity, especially in high-rainfall areas where intensive agriculture and silviculture are most viable. Rates of clearing of TGBs have been very high in recent decades, exceeding rates of tropical forest loss, yet have received little public attention (although see [49]). The Brazilian Cerrado—a hotspot of plant diversity and endemism—has been extensively cleared for agriculture, with more than half lost in the past 50 years, exceeding the rate of forest loss in the Brazilian Amazon [7,50,51]. The TGBs of mainland Southeast Asia and India have been very extensively cleared over the past century [52]. Sub-Saharan and particularly West African savannahs underwent a major phase of agricultural conversion from the mid-1970s, but this had slowed by the 1990s [53,54]. The sparsely populated savannahs of northern Australia represent the largest intact savannah on Earth, with very little land clearing (approx. 1%) having occurred; however, there is an active push by the national government to develop northern Australia for large-scale agriculture [55,56].
Hoekstra et al. [57] identified ‘tropical and subtropical dry broadleaf forests' as the biome that has experienced the greatest rate of historical habitat conversion globally (48.5%). We consider that this biome is largely synonymous with high-rainfall, densely wooded savannahs, largely in mainland Southeast Asia and India, and is hence an example of a TGB (see also [13,58]). It has been suggested that between 35% and more than 60% of the area currently occupied by these biomes are suitable for the development of agriculture [52]. The particular vulnerability of densely wooded TGBs to land-cover change is not surprising as the high rainfall makes them most suitable for agriculture and plantation silviculture, and consequently they have high human population densities (e.g. mainland Southeast Asia and India, figure 1b; Central and West Africa). TGBs in high rainfall areas are likely to be the most species-rich (e.g. figure 4; [35]) and therefore the biodiversity consequences of land-cover conversion are likely to be particularly severe.
Another key threat to the biodiversity of TGBs is woody thickening and forest encroachment, driven by reductions in fire frequency and/or intensity (due to overgrazing, deliberate fire suppression or habitat fragmentation) and increasing atmospheric CO2 concentration [6,10,59,60]. In high-rainfall areas, tropical savannahs can switch to closed forest if disturbance regimes or resource availability are altered [37,61]. The pathway of biodiversity change during such biome shifts remains poorly understood, but if biome shifts occurred at large spatial scales the negative biodiversity impacts would be significant, given that the biomes support such distinct biotas [8,62].
4. Conserving the biodiversity of tropical grassy biomes
We hope that a greater appreciation of the high biodiversity of TGBs will result in a justified increase in the conservation focus on these increasingly threatened biomes. Given the pressure for land-cover conversion, especially in high-rainfall TGBs, networks of large and strategically located protected areas are critical to conserving zones of high-value TGB biodiversity, with resourcing and legal enforcement adequate to: (i) limit land-cover conversion and (ii) maintain critical ecological processes such as fire and grazing. Identification of the biodiversity values of TGBs at a fine spatial scale, and resolving their status as old-growth versus derived, is critical to optimal planning of protected areas.
(a). Tropical grassy biomes in protected areas
Protected areas need to be large if they are to maintain the essential disturbance processes that shape TGBs, and to prevent their transition to more densely woody states. Indeed, the highly fragmented nature of remnant Cerrado in Brazil has severely disrupted ‘natural’ fire regimes, which, combined with a policy of active fire exclusion, has led to widespread increases in the density of trees and shrubs in remnants, threatening endemic species adapted to open, grassy vegetation [63]. Similarly, the need for very large parks to maintain large-scale movements of large migratory herbivores—and the role they play in maintaining woody vegetation cover and its spatial heterogeneity—is already recognized in parts of Africa such as the Serengeti [64,65]. The conservation of many of the iconic predators of TGBs requires very large areas; for example, the persistence of the African wild dog (Lycaon pictus) requires reserves of more than 3500 km2 [66]. Small protected areas in highly fragmented TGB landscapes are likely to require more intensive forms of management to maintain ecological processes critical to biodiversity conservation—such as frequent fire and grazing.
Across the tropics, the proportion of TGBs that are in some form of protected area (13%) is far lower than for forest (24%; figure 1c). However, this discrepancy arises almost entirely because of the large area of protected Neotropical forests and the relatively small area of Neotropical TGBs. In other parts of the tropics, forests and TGBs are afforded proportionally similar levels of protection. This highlights a priority need for a more representative network of protected areas in the Neotropics, where TGB biodiversity and species endemism are particularly high.
Schemes used to prioritize conservation areas are largely based on two axes: vulnerability (e.g. current and potential rates of land-cover conversion) and irreplaceability (e.g. number of endemic species in a region) [67]. The highly influential ‘biodiversity hotspots’ scheme of Myer et al. [47] identifies the Brazilian Cerrado, Madagascar, Mesoamerica and mainland Southeast Asia as regions of highest conservation priority, regardless of biome type. Brooks et al. [67] compared a number of widely used global prioritization schemes and identified areas of the Earth where there was agreement amongst multiple ‘reactive’ schemes (i.e. which target regions in most urgent need of protection). These areas were the hotspots identified by Myer et al. [47], along with India. It is noteworthy that the tropical forests of the Amazon and Congo Basins are not identified by any reactive scheme, primarily because they are considered to be of low vulnerability. It is also noteworthy that, except for the Cerrado, the prevalence of TGBs in these priority regions has only recently been recognized. Many TGBs of mainland Southeast Asia and Mesoamerica are still inappropriately referred to as ‘tropical dry forests’ [41,52], despite recent global-scale maps derived from satellite imagery identifying them as woody savannahs [68].
(b). Valuing ecosystem services provided by tropical grassy biomes
The identification and quantification of appropriate high-value ecosystem services can play an important role in the conservation of TGB biodiversity. A number of researchers have highlighted that carbon schemes (such as the Clean Development Mechanism and Reducing Emissions from Deforestation and Forest Degradation [REDD+]) can be a threat if they promote tree planting in old-growth grasslands [6,10,59,69]. However, with appropriate safeguards to avoid perverse biodiversity outcomes (e.g. disallowing afforestation), carbon schemes can help maintain high-biomass savannahs in biodiverse, high-rainfall regions (e.g. [27,70]). A key to using carbon schemes to encourage the retention of high-biomass TGBs is an improved understanding of the distribution of natural and anthropogenically derived TGBs, their carbon-storage potential and how this interacts with biodiversity values (e.g. [71]).
Another high-value ecosystem service provided by relatively intact TGBs is wildlife-based tourism, including safari-hunting. It has been shown that the income potentially derived from ‘ecotourism’ exceeds that from replacement of native vegetation with cash crops [72]. In sub-Saharan Africa, safari-hunting in TGBs brings in many tens of millions of US dollars annually, and much of the hunting occurs in private or communally owned hunting reserves [73]. In a recent analysis of the income earned by communal conservancies in Namibia, the greatest economic benefits were obtained from a mix of hunting and ‘photographic’ tourism [74]. TGBs typically provide better opportunities for both hunting and viewing charismatic megafauna than dense forests, so ecotourism is likely to provide a relatively strong economic incentive to retain TGBs. Ecotourism may also provide an incentive to prevent woody thickening in TGBs, as it can significantly reduce opportunities for game viewing and therefore diminish visitor satisfaction [75].
5. Conclusion
The plight faced by tropical forests has captured public attention for decades, yet TGBs have not enjoyed such concern despite supporting outstanding biodiversity values and facing similar rates of habitat loss. There has been a widespread misconception that TGBs are anthropogenically degraded forests, and only now is there an emerging appreciation of biodiverse old-growth TGBs, worthy of a focused conservation effort. We have used an analysis of globally consistent datasets of vertebrate species richness to show that, once effects of biogeographic realm, rainfall and latitude are accounted for, there is little difference in local vertebrate species richness between TGBs and tropical forest. The pattern for vascular plants was somewhat different, with TGBs having significantly lower species richness than tropical forests. Clearly, the simplistic notion that TGBs have low biodiversity is not valid.
TGBs have a critical role to play in biodiversity conservation globally. Those in the Neotropics stand out as being among the most biodiverse on Earth, and a number of these are considered global ‘biodiversity hotspots’ [47], with high endemic biodiversity threatened by high rates of land-cover conversion, including Brazilian Cerrado and the savannah forests of Mesoamerica. Extensive TGBs also occur in the biodiversity hotspots of Southeast Asia and Madagascar. The high-rainfall TGBs of the Afrotropics ranked highly in terms of biodiversity, yet rates of land-cover conversion have been historically low. Demand for agricultural products, including biofuels, is likely to put pressure on African TGBs in coming decades [27,76,77].
The policies and management actions required to conserve TGB biodiversity will vary throughout the tropics. In line with varied threats, there is no ‘one size fits all’ approach to the management of TGBs; a management paradigm that works in one region should not be unquestioningly applied elsewhere. However, the key to conserving TGBs is a wider recognition—among conservation scientists, policy-makers and the general public—that TGBs are globally important stores of biodiversity and worthy of a focused conservation effort. A key research priority must be to clarify the true distribution of TGBs across the tropics, including the distinction between ancient and derived TGBs, and between densely wooded savannahs and dry forests.
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
The full dataset used in the analysis of species richness across biomes and biogeographic realms is provided in the electronic supplementary material, appendix S1.
Authors' contributions
B.P.M. and C.L.P. conceived the manuscript; B.P.M. carried out the analysis and led the writing; all authors contributed to the writing and reviewed early versions of the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
B.P.M. was supported by a fellowship from the Australian Research Council (DE130100434), and the National Environmental Science Programme's Threatened Species Recovery Hub.
References
- 1.Udvardy M. 1975. A classification of the biogeographical provinces of the world. Morges, Switzerland: International Union for Conservation of Nature. [Google Scholar]
- 2.Center for International Earth Science Information Network - CIESIN - Columbia University, a.C.I.d.A.T.-C. 2005. Gridded Population of the World, Version 3 (GPWv3): Population Density Grid. Palisades, NY: NASA Socioeconomic Data and Applications Center. [Google Scholar]
- 3.World Conservation Union and UNEP World Conservation Monitoring Centre. 2016. World database on protected areas. Cambridge, UK: UNEP World Conservation Monitoring Centre. [Google Scholar]
- 4.IUCN and UNEP-WCMC. 2016. The world database on protected areas (WDPA). Cambridge, UK: UNEP World Conservation Monitoring Centre. [Google Scholar]
- 5.Jenkins CN, Pimm SL, Joppa LN. 2013. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl Acad. Sci. USA 110, E2602–E2610. ( 10.1073/pnas.1302251110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parr CL, Lehmann CER, Bond WJ, Hoffmann WA, Andersen AN. 2014. Tropical grassy biomes: misunderstood, neglected, and under threat. Trends Ecol. Evol. 29, 205–213. ( 10.1016/j.tree.2014.02.004) [DOI] [PubMed] [Google Scholar]
- 7.Overbeck GE, et al. 2015. Conservation in Brazil needs to include non-forest ecosystems. Divers. Distrib. 21, 1455–1460. ( 10.1111/ddi.12380) [DOI] [Google Scholar]
- 8.Bond WJ, Parr CL. 2010. Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biol. Conserv. 143, 2395–2404. ( 10.1016/j.biocon.2009.12.012) [DOI] [Google Scholar]
- 9.Veldman JW, Putz FE. 2011. Grass-dominated vegetation, not species-diverse natural savanna, replaces degraded tropical forests on the southern edge of the Amazon Basin. Biol. Conserv. 144, 1419–1429. ( 10.1016/j.biocon.2011.01.011) [DOI] [Google Scholar]
- 10.Bond WJ. 2016. Ancient grasslands at risk. Science 351, 120–122. ( 10.1126/science.aad5132) [DOI] [PubMed] [Google Scholar]
- 11.Veldman JW, et al. . 2015. Toward an old-growth concept for grasslands, savannas, and woodlands. Front. Ecol. Environ. 13, 154–162. ( 10.1890/140270) [DOI] [Google Scholar]
- 12.Vorontsova MS, et al. 2016. Madagascar's grasses and grasslands: anthropogenic or natural? Proc. R. Soc. B 283, 20152262 ( 10.1098/rspb.2015.2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratnam J, Bond WJ, Fensham RJ, Hoffmann WA, Archibald S, Lehmann CER, Anderson MT, Higgins SI, Sankaran M. 2011. When is a ‘forest’ a savanna, and why does it matter? Glob. Ecol. Biogeogr. 20, 653–660. ( 10.1111/j.1466-8238.2010.00634.x) [DOI] [Google Scholar]
- 14.Noss RF. 2012. Forgotten grasslands of the south: natural history and conservation. Washington, DC: Island Press. [Google Scholar]
- 15.Andersen AN, Arnan X, Sparks K. 2013. Limited niche differentiation within remarkable co-occurrences of congeneric species: monomorium ants in the Australian seasonal tropics. Aust. Ecol. 38, 557–567. ( 10.1111/aec.12000) [DOI] [Google Scholar]
- 16.Brühl CA, Gunsalam G, Linsenmair KE. 1998. Stratification of ants (Hymenoptera, Formicidae) in a primary rain forest in Sabah, Borneo. J. Trop. Ecol. 14, 285–297. ( 10.1017/S0266467498000224) [DOI] [Google Scholar]
- 17.Lehmann CER, et al. 2014. Savanna vegetation-fire-climate relationships differ among continents. Science 343, 548–552. (doi:.10.1126/science1247355) [DOI] [PubMed] [Google Scholar]
- 18.Moncrieff GR, Lehmann CER, Schnitzler J, Gambiza J, Hiernaux P, Ryan CM, Shackleton CM, Williams RJ, Higgins SI. 2014. Contrasting architecture of key African and Australian savanna tree taxa drives intercontinental structural divergence. Glob. Ecol. Biogeogr. 23, 1235–1244. ( 10.1111/geb.12205) [DOI] [Google Scholar]
- 19.Campos RI, Vasconcelos HL, Andersen AN, Frizzo TL, Spena KC. 2011. Multi-scale ant diversity in savanna woodlands: an intercontinental comparison. Aust. Ecol. 36, 983–992. ( 10.1111/j.1442-9993.2011.02255.x) [DOI] [Google Scholar]
- 20.Corlett RT, Primack RB. 2006. Tropical rainforests and the need for cross-continental comparisons. Trends Ecol. Evol. 21, 104–110. ( 10.1016/j.tree.2005.12.002) [DOI] [PubMed] [Google Scholar]
- 21.Pearson DL. 1977. A pantropical comparison of bird community structure on six lowland forest sites. The Condor 79, 232–244. ( 10.2307/1367167) [DOI] [Google Scholar]
- 22.Bond WJ, Cook GD, Williams RJ. 2012. Which trees dominate in savannas? The escape hypothesis and eucalypts in northern Australia. Aust. Ecol. 37, 678–685. ( 10.1111/j.1442-9993.2011.02343.x) [DOI] [Google Scholar]
- 23.Murphy BP, Liedloff AC, Cook GD. 2015. Does fire limit tree biomass in Australian savannas? Int. J. Wildland Fire 24, 1–13. ( 10.1071/WF14092) [DOI] [Google Scholar]
- 24.Leal IR, Wirth R, Tabarelli M. 2014. The multiple impacts of leaf-cutting ants and their novel ecological role in human-modified Neotropical forests. Biotropica 46, 516–528. ( 10.1111/btp.12126) [DOI] [Google Scholar]
- 25.Linder HP. 2014. The evolution of African plant diversity. Front. Ecol. Evol. 2, 38 ( 10.3389/fevo.2014.00038) [DOI] [Google Scholar]
- 26.Simon MF, Grether R, de Queiroz LP, Skema C, Pennington RT, Hughes CE. 2009. Recent assembly of the Cerrado, a Neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl Acad. Sci. USA 106, 20 359–20 364. ( 10.1073/pnas.0903410106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Searchinger TD, Estes L, Thornton PK, Beringer T, Notenbaert A, Rubenstein D, Heimlich R, Licker R, Herrero M. 2015. High carbon and biodiversity costs from converting Africa's wet savannahs to cropland. Nat. Clim. Change 5, 481–486. ( 10.1038/nclimate2584) [DOI] [Google Scholar]
- 28.Andersen AN, Del Toro I, Parr CL. 2015. Savanna ant species richness is maintained along a bioclimatic gradient of increasing latitude and decreasing rainfall in northern Australia. J. Biogeogr. 42, 2313–2322. ( 10.1111/jbi.12599) [DOI] [Google Scholar]
- 29.Andersen AN, Hertog T, Woinarski JCZ. 2006. Long-term fire exclusion and ant community structure in an Australian tropical savanna: congruence with vegetation succession. J. Biogeogr. 33, 823–832. ( 10.1111/j.1365-2699.2006.01463.x). [DOI] [Google Scholar]
- 30.Reichel H, Andersen AN. 1996. The rainforest ant fauna of Australia's Northern Territory. Aust. J. Zool. 44, 81–95. ( 10.1071/ZO9960081) [DOI] [Google Scholar]
- 31.Kier G, Kreft H, Lee TM, Jetz W, Ibisch PL, Nowicki C, Mutke J, Barthlott W. 2009. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl Acad. Sci. USA 106, 9322–9327. ( 10.1073/pnas.0810306106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentry AH. 1988. Tree species richness of upper Amazonian forests. Proc. Natl Acad. Sci. USA 85, 156–159. ( 10.1073/pnas.85.1.156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turpie JK, Crowe TM. 1994. Patterns of distribution, diversity and endemism of larger African mammals. S Afr. J. Zool. 29, 19–32. ( 10.1080/02541858.1994.11448322) [DOI] [Google Scholar]
- 34.Anderson TM, White S, Davis B, Erhardt R, Palmer M, Swanson A, Kosmala M, Packer C. 2016. The spatial distribution of African savannah herbivores: species associations and habitat occupancy in a landscape context. Phil. Trans. R. Soc. B 371, 20150314 ( 10.1098/rstb.2015.0314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kier G, Mutke J, Dinerstein E, Ricketts TH, Küper W, Kreft H, Barthlott W. 2005. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 32, 1107–1116. ( 10.1111/j.1365-2699.2005.01272.x) [DOI] [Google Scholar]
- 36.Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117. ( 10.1890/03-8006) [DOI] [Google Scholar]
- 37.Murphy BP, Bowman DMJS. 2012. What controls the distribution of tropical forest and savanna? Ecol. Lett. 15, 748–758. ( 10.1111/j.1461-0248.2012.01771.x) [DOI] [PubMed] [Google Scholar]
- 38.Hirota M, Holmgren M, Van Nes EH, Scheffer M. 2011. Global resilience of tropical forest and savanna to critical transitions. Science 334, 232–235. ( 10.1126/science.1210657) [DOI] [PubMed] [Google Scholar]
- 39.van Nes EH, Hirota M, Holmgren M, Scheffer M. 2014. Tipping points in tropical tree cover: linking theory to data. Glob. Change Biol. 20, 1016–1021. ( 10.1111/gcb.12398) [DOI] [PubMed] [Google Scholar]
- 40.Bond WJ, Woodward FI, Midgley GF. 2005. The global distribution of ecosystems in a world without fire. New Phytol. 165, 525–538. ( 10.1111/j.1469-8137.2004.01252.x) [DOI] [PubMed] [Google Scholar]
- 41.Olson DM, et al. 2001. Terrestrial ecoregions of the world: a new map of life on Earth. BioScience 51, 933–938. ( 10.1641/0006-3568(2001)051%5B0933:teotwa%5D2.0.co;2) [DOI] [Google Scholar]
- 42.Köppen W. 1931. Grundriss der Klimakunde. Berlin, Germany: De Gruyter. [Google Scholar]
- 43.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276). [DOI] [Google Scholar]
- 44.Ratnam J, Tomlinson KW, Rasquinha DN, Sankaran M. 2016. Savannahs of Asia: antiquity, biogeography, and an uncertain future. Phil. Trans. R. Soc. B 371, 20150305 ( 10.1098/rstb.2015.0305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 46.Crawley MJ. 2012. The R Book. Chichester, UK: John Wiley and Sons. [Google Scholar]
- 47.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. (http://www.nature.com/nature/journal/v403/n6772/suppinfo/403853a0_S1.html) [DOI] [PubMed] [Google Scholar]
- 48.Lehmann CER, Archibald SA, Hoffmann WA, Bond WJ. 2011. Deciphering the distribution of the savanna biome. New Phytol. 191, 197–209. ( 10.1111/j.1469-8137.2011.03689.x) [DOI] [PubMed] [Google Scholar]
- 49.Espírito-Santo MM, Leite ME, Silva JO, Barbosa RS, Rocha AM, Anaya FC, Dupin MGV. 2016. Understanding patterns of land-cover change in the Brazilian Cerrado from 2000 to 2015. Phil. Trans. R. Soc. B 371, 20150435 ( 10.1098/rstb.2015.0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratter JA, Ribeiro JF, Bridgewater S. 1997. The Brazilian Cerrado vegetation and threats to its biodiversity. Ann. Bot. 80, 223–230. ( 10.1006/anbo.1997.0469) [DOI] [Google Scholar]
- 51.Beuchle R, Grecchi RC, Shimabukuro YE, Seliger R, Eva HD, Sano E, Achard F. 2015. Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Appl. Geography 58, 116–127. ( 10.1016/j.apgeog.2015.01.017) [DOI] [Google Scholar]
- 52.Miles L, Newton AC, DeFries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE. 2006. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 33, 491–505. ( 10.1111/j.1365-2699.2005.01424.x) [DOI] [Google Scholar]
- 53.Brink AB, Eva HD. 2009. Monitoring 25 years of land cover change dynamics in Africa: a sample based remote sensing approach. Appl. Geography 29, 501–512. ( 10.1016/j.apgeog.2008.10.004) [DOI] [Google Scholar]
- 54.Gibbs HK, Ruesch AS, Achard F, Clayton MK, Holmgren P, Ramankutty N, Foley JA. 2010. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl Acad. Sci. USA 107, 16 732–16 737. ( 10.1073/pnas.0910275107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Commonwealth of Australia 2015. Our north, our future: white paper on developing Northern Australia. Canberra, Australia: Commonwealth of Australia. [Google Scholar]
- 56.Northern Australia Land and Water Taskforce 2010. Sustainable development in Northern Australia: A report to government from the Northern Australia Land and Water Taskforce. Canberra, Australia: Australian Department of Infrastructure, Transport, Regional Development and Local Government. [Google Scholar]
- 57.Hoekstra JM, Boucher TM, Ricketts TH, Roberts C. 2005. Confronting a biome crisis: global disparities of habitat loss and protection. Ecol. Lett. 8, 23–29. ( 10.1111/j.1461-0248.2004.00686.x) [DOI] [Google Scholar]
- 58.Dexter K, et al. 2015. Floristics and biogeography of vegetation in seasonally dry tropical regions. Int. Forestry Rev. 17, 10–32. ( 10.1505/146554815815834859) [DOI] [Google Scholar]
- 59.Veldman JW, et al. 2015. Tyranny of trees in grassy biomes. Science 347, 484–485. ( 10.1126/science.347.6221.484-c) [DOI] [PubMed] [Google Scholar]
- 60.Durigan G, Ratter JA. 2016. The need for a consistent fire policy for Cerrado conservation. J. Appl. Ecol. 53, 11–15. ( 10.1111/1365-2664.12559) [DOI] [Google Scholar]
- 61.Wigley BJ, Bond WJ, Hoffman MT. 2010. Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob. Change Biol. 16, 964–976. ( 10.1111/j.1365-2486.2009.02030.x) [DOI] [Google Scholar]
- 62.Parr CL, Gray EF, Bond WJ. 2012. Cascading biodiversity and functional consequences of a global change-induced biome switch. Divers. Distrib. 18, 493–503. ( 10.1111/j.1472-4642.2012.00882.x) [DOI] [Google Scholar]
- 63.Durigan G, Ratter JA. 2006. Successional changes in Cerrado and Cerrado/forest ecotonal vegetation in western São Paulo State, Brazil, 1962–2000. Edinb. J. Bot. 63, 119–130. ( 10.1017/S0960428606000357) [DOI] [Google Scholar]
- 64.Holdo RM, Holt RD, Fryxell JM. 2009. Grazers, browsers, and fire influence the extent and spatial pattern of tree cover in the Serengeti. Ecol. Appl. 19, 95–109. ( 10.1890/07-1954.1) [DOI] [PubMed] [Google Scholar]
- 65.Fryxell JM, Wilmshurst JF, Sinclair ARE, Haydon DT, Holt RD, Abrams PA. 2005. Landscape scale, heterogeneity, and the viability of Serengeti grazers. Ecol. Lett. 8, 328–335. ( 10.1111/j.1461-0248.2005.00727.x) [DOI] [Google Scholar]
- 66.Woodroffe R, Ginsberg JR. 1998. Edge effects and the extinction of populations inside protected areas. Science 280, 2126–2128. ( 10.1126/science.280.5372.2126) [DOI] [PubMed] [Google Scholar]
- 67.Brooks TM, Mittermeier RA, da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL. 2006. Global biodiversity conservation priorities. Science 313, 58–61. ( 10.1126/science.1127609) [DOI] [PubMed] [Google Scholar]
- 68.Friedl MA, Sulla-Menashe D, Tan B, Schneider A, Ramankutty N, Sibley A, Huang X. 2010. MODIS Collection 5 Global Land Cover: Algorithm Refinements and Characterization of New Datasets, 2001–2012, Collection 5.1 IGBP Land Cover. Boston, MA: Boston University. [Google Scholar]
- 69.Lehmann CER. 2010. Savannas need protection. Science 327, 642–643. ( 10.1126/science.327.5966.642-c) [DOI] [PubMed] [Google Scholar]
- 70.Van Oosterzee P, Garnett ST. 2008. Seeing REDD: issues, principles and possible opportunities in Northern Australia. Public Adminis. Dev. 28, 386–392. ( 10.1002/pad.511) [DOI] [Google Scholar]
- 71.Pellegrini AFA, Socolar JB, Elsen PR, Giam X. In press Tradeoffs between savanna woody plant diversity and carbon storage in the Brazilian Cerrado. Glob. Change Biol. ( 10.1111/gcb.13259) [DOI] [PubMed] [Google Scholar]
- 72.Naidoo R, Adamowicz WL. 2005. Economic benefits of biodiversity exceed costs of conservation at an African rainforest reserve. Proc. Natl Acad. Sci. USA 102, 16 712–16 716. ( 10.1073/pnas.0508036102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Minin E, Leader-Williams N, Bradshaw CJA. 2016. Banning trophy hunting will exacerbate biodiversity loss. Trends Ecol. Evol. 31, 99–102. ( 10.1016/j.tree.2015.12.006) [DOI] [PubMed] [Google Scholar]
- 74.Naidoo R, Weaver LC, Diggle RW, Matongo G, Stuart-Hill G, Thouless C. 2016. Complementary benefits of tourism and hunting to communal conservancies in Namibia. Conserv. Biol. 30, 628–638. ( 10.1111/cobi.12643) [DOI] [PubMed] [Google Scholar]
- 75.Gray EF, Bond WJ. 2013. Will woody plant encroachment impact the visitor experience and economy of conservation areas? Koedoe 55, 1–9. ( 10.4102/koedoe.v55i1.1106) [DOI] [Google Scholar]
- 76.Morris ML, Binswanger-Mikhize HP, Byerlee D. 2009. Awakening Africa's sleeping giant: prospects for commercial agriculture in the guinea savannah zone and beyond. Washington, DC: World Bank Publications. [Google Scholar]
- 77.Roxburgh C, et al. 2010. Lions on the move: the progress and potential of African economies. Washington, DC: McKinsey Global Institute. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset used in the analysis of species richness across biomes and biogeographic realms is provided in the electronic supplementary material, appendix S1.