Abstract
Introduction:
Adequate evaluation of novel tobacco products must include investigation of consumers’ psychological response to such products. Traditionally, subjective scales of product liking have been used to assess consumer acceptability of tobacco products. However, subjective scales may miss cognitive changes that can only be captured by direct neurophysiological assessment. The present investigation explored the viability of using electroencephalography (EEG), in combination with traditional subjective measures, to assess consumer acceptability of five smokeless tobacco products. Given previous work linking product liking to arousal/attentional (executive function) enhancement, we focused on EEG measures of attention/arousal to objectively characterize cognitive changes associated with tobacco product use.
Methods:
During five separate laboratory visits, smokeless tobacco users used Verve discs, Ariva dissolvables, Skoal snuff, Camel snus, or Nicorette lozenges. The N2 and P3b event-related potential components elicited by an oddball task were used to index attentional changes before/after product usage. Additionally, resting state alpha band EEG activity was analyzed before/after product usage to index cortical arousal.
Results:
Although analyses of the subjective results provided limited inference, analyses of the electrophysiological measures, particularly the alpha suppression measure, revealed robust differences between products. Skoal elicited significantly enhanced alpha suppression compared to all four other products tested. Additionally, alpha suppression was found to correlate positively with subjective measures of satisfaction and psychological reward, but was unrelated to perceived aversion.
Conclusions:
The present results provide evidence that electrophysiological measures can yield important insights into consumer acceptability of novel tobacco products and are a valuable complement to subjective measures.
Implications:
This study is the first to employ a combination of electrophysiological measures and traditional subjective assays in order to assess the consumer acceptability of smokeless tobacco products. The results highlight the importance of adopting a multidimensional/multi-method approach to studying the consumer acceptability of tobacco products.
Introduction
Effective regulation of novel tobacco products relies on strong scientific evidence to inform policy makers. It is well known that the adverse effects of tobacco product toxicity are directly related to the extent and duration of product use.1 A recent model suggests that adoption and long term use of novel tobacco products is influenced by perceptions of the product (in turn influenced by advertising, social norms, perceived safety, etc.) as well as the direct product response (sensory effects, satisfaction, psychological reward, etc).2 Therefore, in addition to consideration of product toxicity, the scientific assessment of consumer acceptability is an essential component of tobacco product evaluation.
Traditionally, assessment of smoked tobacco products, and more recently smokeless tobacco products,3–5 has relied primarily on subjective evaluations to inform measures of consumer acceptability. Various subjective reporting methods have long and well-validated histories in determining product satisfaction,6 as well as a given product’s ability to diminish smoking urges.7 While useful, subjective reports may be limited in their ability to fully the capture consumers’ response to novel tobacco products. For example, it has previously been demonstrated that the ability of a tobacco product to alter executive function, such as attention/arousal (also referred to as “cognitive control”), is directly related to product use.8–11 That is, one of the hallmarks of a tobacco product that is deemed “acceptable” to consumers is the ability of the product to enhance executive control. However, direct measures of executive control are not typically employed when assessing the consumer acceptability of novel tobacco products. Therefore, neurocognitive assessments, such as electroencephalography (EEG), that provide objective measurements of specific cognitive functions may provide an ideal complement to traditional subjective measures in evaluating tobacco products.
Scalp-recorded EEG provides a noninvasive and cost-effective method to assess brain function in real time. EEG can measure specific cognitive processes, such as attention and arousal, which have been linked to tobacco use and abuse.8–11 When EEG is recorded during a target detection task, target detection is typically associated with two event-related potential (ERP) components: the N2 and P3b components. In visual tasks, N2 is believed to reflect the allocation of attention toward sensory information.12 The P3b is also believed to be influenced by attention, but additionally reflects higher-order processes such the updating of working memory,13 the outcome of decision processes,14 or the decision process itself.15 EEG recordings when participants are not engaged in a task, but in a “resting state,” have also proven useful for delineating cognitive function. For example, it’s well established that EEG alpha band power (8–12 Hz) is negatively correlated with arousal levels.16,17 Given that one of the most commonly reported reasons individuals use tobacco is to improve attention and arousal,8–11 assessing N2, P3b, and alpha suppression before and after product usage may prove useful in objectively identifying which tobacco products elicit a preferential response. In line with this notion, previous research has shown that traditional cigarettes, or nicotine alone, can enhance alpha suppression,18–21 N2,22 and P3b amplitude.22–24
EEG and ERPs have previously been used as a way to assess the effect of tobacco or nicotine on attention and brain state arousal.18,23,25–30 However, to our knowledge, this is the first study employing EEG in conjunction with traditional subjective measures of consumer acceptability in order to compare a variety of smokeless tobacco products. Given recent interest in using some smokeless tobacco products as possible harm reduction products,31 the present attempt to provide a more extensive comparison of smokeless tobacco products provides an important addition to the literature. The current study assessed N2, P3b, and alpha suppression before and after use of five smokeless tobacco products. Additionally, traditional subjective measures were assessed. The primary aim of the present investigation was to explore the viability of using a combination of subjective and electrophysiological measures as tools to separate smokeless products based on their consumer acceptability. Whereas subjective measures were used to assess known cognitive constructs related to consumer acceptability, electrophysiology was used to objectively quantify changes in arousal/attention which have been linked to product use,8–11 but that are not typically assessed. Given that attention/arousal is related to product liking, we would expect the electrophysiological and subjective measures to be partially correlated with one another. However, rather than assessing the same underlying construct, the electrophysiological and subjective measures are believed to assess distinct, but complementary, aspects of consumer acceptability. Thus, we employed a multidimensional/multi-method approach to studying consumer acceptability.
Methods
Participants
Thirty male smokeless tobacco users (28 Caucasian, one African American and one Asian American) ranging in age from 19 to 61 years (M age = 22.2 years) were paid to participate in the study. All participants reported using smokeless tobacco for at least 1 year prior to study participation. All participants were right-handed, were not currently taking any medications known to affect the central nervous system and reported no use of illicit drugs. All participants provided written informed consent and all procedures were approved by the Institutional Review Board of the University of Maryland, College Park.
Procedure
On five separate laboratory visits, participants were exposed to one of four smokeless tobacco products (Camel snus, Verve chewable disc, Skoal snuff, Ariva dissolvable tablet) or a Nicorette nicotine lozenge as a control (see section entitled “smokeless tobacco products”). Each laboratory visit occurred on a separate day between 9 AM–5 PM. Participants were naïve to the product administered on each visit and product order was counterbalanced across participants. Participants were instructed to abstain from tobacco use for 12 hours and alcohol use for 24 hours prior to each laboratory visit.
Laboratory visits began with participants completing a series of subjective scales (see “subjective scales” section). While completing the subjective scales, an EEG cap was placed on the participant’s head (see “electrophysiological recording” section). Participants then practiced and completed an oddball cognitive task while EEG was recorded (see “cognitive task” section). Participants were then provided with the smokeless tobacco product and instructed on its use. Prior to beginning product usage, a 90-second baseline EEG recording was taken. Participants then began product usage while watching a nature documentary. Product usage continued for 30 minutes, with 90-second resting state EEG recordings taken every 6 minutes. During each EEG recording the nature documentary was turned off; participants were asked to maintain visual fixation and limit movement and eye blinks. In total, six resting-state EEG recordings were made: a baseline recording (immediately prior to product usage), as well as 6, 12, 18, 24, and 30 minutes after the initiation of product usage. We chose to record resting state EEG with eyes open, given that resting state EEG was recorded at several time points during a 30-minute period in which the subjects’ eyes remained open. There was a concern that having subjects close their eyes prior to each EEG resting state recording might actually reduce cortical arousal. Given previous work by Barry and colleagues16,17 suggesting that both eyes-open and eyes-closed EEG can be used to assess arousal, we chose to employ an eyes-open methodology for the current investigation. Following product usage, any remaining product was removed from the mouth and participants completed the oddball task a second time while EEG was recorded. Participants then completed a subset of the subjective scales a second time prior to leaving the laboratory (see “subjective scales” section).
Smokeless Tobacco Products
The following five products were administered to participants on separate visits: Camel snus “mellow” flavor (unprotonated nicotine = 1.25mg, total = 4.96mg), Ariva dissolvable tablet “wintergreen” flavor (unprotonated nicotine = 0.067mg, total = 1.30mg), Verve chewable disc “blue mint” flavor (unprotonated nicotine = 0.24mg, total = 0.90mg), Skoal snuff “classic straight” flavor (unprotonated nicotine = 3.27mg, total = 15.4mg), Nicorette nicotine lozenge “original” flavor (unprotonated nicotine = 1.70mg, total = 1.73mg).
Subjective Scales
The following questionnaires were completed on the first laboratory visit only: the demographic and tobacco use history questionnaire, and a modified version of the Fägerstrom Test for Nicotine Dependence (mFTND). A modified version of the Questionnaire on Smoking urges (mQSU-brief)7 was completed twice on each laboratory visit (before/after product usage), while a modified version of the Cigarette Evaluation Scale (mCES),6 also referred to as the “Cigarette Evaluation Questionnaire,”32 was completed on each laboratory visit following product usage. Where appropriate, questionnaires were modified for assessing smokeless tobacco products (eg, “smoke cigarettes” changed to “use smokeless tobacco”).
Cognitive Task
The cognitive task was a visual “oddball” task; participants responded to infrequently presented targets, while withholding responses to frequently presented nontargets. Trials consisted of either a target “X” or nontarget “O” presented in the center of an LCD monitor for 100ms using Presentation software (Neurobehavioral Systems, California). Participants sat approximately 100cm away from the monitor and stimuli subtended approximately 1.72 by 1.72° visual angle. Participants responded to target stimuli with the left button of a computer mouse. Targets were presented with a 25% probability and trials were separated by an inter-stimulus-interval jittered between 1000–1500ms. Participants completed two blocks (150 trials/block) of the task before and after product usage (~7 minutes).
Electrophysiological Recording
EEG was recorded from 16 scalp sites (FP1, FP2, F3, Fz, F4, T3, C3, Cz, C4, T4, P3, Pz, P4, O1, Oz, O2) with Ag/AgCl electrodes embedded in an elastic cap (extended 10–20 system). Electrodes were placed at the left supraorbital and suborbital sites, and left and right outer canthal sites, to monitor vertical and horizontal electro-oculographic (EOG) activity. Data was sampled at 500 Hz using a Neuroscan NuAmps amplifier and SCAN 4.01 software (Compumedics, North Carolina). All scalp electrodes were referenced to the left mastoid on-line and re-referenced to the average of the left and right mastoids offline. All electrode impedances were maintained below 10kΩ and recorded with a 70-Hz low-pass filter.
Analysis of Subjective Scales
Descriptive statistics (mean and standard error) were calculated for the mFTND questionnaire responses. For the mCES, scores on the “psychological reward,” “satisfaction” and “aversion” multiple item factors were calculated for each product/participant, following product usage.32 Although the mCES also allows for the calculation of an “enjoy sensations” and a “craving reduction” factor, these factors are only based on single items and were not analyzed for the current report. For the mQSU-brief, scores on the “relief from withdrawal” and “intention to use smokeless tobacco” factors were calculated for each product/participant, before/after product usage7; difference scores for each factor (post minus pre) were then calculated for each product/participant. For each factor of the mCES and mQSU-brief, a separate repeated-measures analysis of variance (ANOVA) model to test mean differences between the product types was used. For all ANOVAs, P values were adjusted for multiple pairwise comparisons using a Bonferroni correction.
Analysis of Behavioral Data From Cognitive Task
Target accuracy and response time was calculated for each product/participant, before/after product usage; difference scores for target accuracy and response time (post minus pre) were then calculated for each product/participant. For both accuracy and response time, separate repeated measures ANOVA models were used to test mean differences between the product types.
Analysis of Electrophysiological Data
Preprocessing
Following acquisition, data from in-cap electrodes was re-referenced to the average of the left/right mastoid recordings and linearly detrended to remove large linear drifts. Data was low-pass filtered at 30 Hz, using a Butterworth filter.33 Data from the oddball task was epoched into stimulus-locked epochs (−150 to 800ms relative to stimulus onset), whereas resting state EEG data was epoched into 1000ms epochs. An initial rejection of large artifacts (such as electrode pop-offs) were rejected using a ±1000 µV rejection threshold. Large linear drifts within epochs were rejected using the pop_rejtrend function of the EEGLAB toolbox, with a maximum slope threshold of 75 µV and an R 2 limit of 0.8.34 Epochs containing EMG-like activity were rejected using the EEGLAB pop_rejspec function, using a 50 dB threshold within the 20–40 Hz band.34 To remove ocular artifacts and additional noise, independent component analysis decomposition was run on an identical dataset with the addition of a 1-Hz high-pass filter.35 Identified independent component analysis components were copied to the original data set and rejected. Stimulus-locked epochs were baseline corrected to the 150ms prestimulus baseline period. A final threshold rejection of ±50 µV was performed to remove remaining artifactual activity in the resting state EEG and stimulus-locked epochs. Following artifact rejection and removal of participants for incomplete data, 22 participants remained for the ERP analysis and 23 participants remained for the EEG analysis.
ERP Analysis
Only correct trials were analyzed. For N2 and P3b, the time window and electrode locations used for ERP quantification was based on previous literature and inspection of the grand-average ERP waveforms collapsed across all conditions. For N2, mean amplitudes were extracted from the 236–276ms time window at electrodes O1 and O2 and collapsed across electrode location. For P3b, mean amplitudes were extracted from the 380–420ms time window, at electrode Pz. To compare how the usage of each product influenced N2 and P3b, difference scores (post minus pre) were calculated for each ERP component. Specifically, the mean amplitude extracted from the oddball task completed before product usage was subtracted from the mean amplitude extracted from the oddball task after product usage. The N2 and P3b difference scores were analyzed using a repeated measures ANOVA model to test mean differences between product types.
EEG Analysis
Resting state EEG epochs were linearly detrended using the MATLAB function detrend, then transformed to power spectral density (PSD) via a 512-point hamming-windowed Fourier transform (frequency bin width = 0.98 Hz). Raw PSD (μV2/Hz) was logarithmically transformed to decibel (dB) PSD (10 × log10 [μV2/Hz]). Global alpha power was defined as PSD between bin centers 7.81 Hz and 12.70 Hz at electrodes F3, F4, C3, C4, P3, and P4. For each resting state period, global alpha power was averaged across epochs. To investigate how each product influenced global alpha power over time, alpha difference scores were calculated for each time point/product. Specifically, global alpha power before product usage was subtracted from global alpha power for each time point after usage. Changes in global alpha power, as a function of product type and time point, were analyzed using a repeated measures ANOVA model to test mean differences between the product types and time points.
Analyses of Relationships Between Electrophysiology and Subjective Scales
Potential correlations between the physiological measures and the subjective scales were tested. Separate Pearson product-moment correlations were calculated for the N2, P3b, and global alpha difference scores with the following: means of the “psychological reward,” “satisfaction” and “aversion” factors of the mCES, and the “relief from withdrawal” and “intention to use smokeless tobacco” factors of the mQSU-brief. To improve interpretability of these correlations, the sign of the N2 and alpha power difference scores were reversed (ie, an increased N2 or increased alpha suppression would now register as a positive value).
Results
Demographic Information
On average, participants had been using smokeless tobacco at a rate of 1.7 tins or 8.9 pouches/lozenges per week for a mean of 21.72 months (SD = 15.01 months) and had a mean mFTND score of 1.27 (SD = 1.23). The level of smokeless tobacco use among participants in this study can be considered as a light level of dependence, suggesting that the effect of smokeless tobacco use in the present sample was less influenced by withdrawal-reduction. Of the 30 smokeless tobacco users included in this study, five were also current cigarette smokers (smoking four or less cigarettes per day), 21 were previous cigarette smokers, and four had never used cigarettes.
Subjective Results
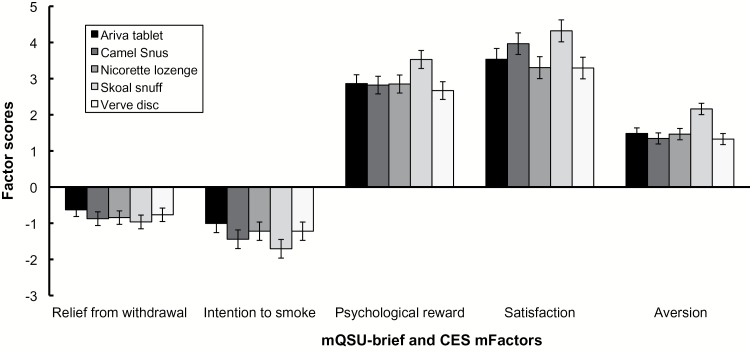
Subjective analyses were conducted for all 30 participants (see Figure 1). Analysis of the mQSU-brief yielded no significant effect of product type for either the “relief from withdrawal” (P = .514) or “intention to use smokeless tobacco” (P = .138) factors. Analysis of the mCES “satisfaction” factor revealed a main effect of product type (F (4,109) = 3.13, P = .018). However, none of the follow-up comparisons remained significant after multiple comparisons correction. Analysis of the mCES “psychological reward” factor revealed a main effect of product type (F (4,109) = 2.94, P = .024). Follow-up paired comparisons revealed that Skoal was rated higher in psychological reward than Verve (corrected P = .023). Analysis of the mCES “aversion” factor revealed a main effect of product type (F (4,109) = 7.10, P = .0001). Follow-up paired comparisons revealed that Skoal was rated higher in aversion than Ariva (corrected P = .003), Snus (corrected P = .0002), Verve (corrected P = .002), and Nicorette (corrected P = .0001).
Figure 1.
Factor scores for modified version of the Questionnaire on Smoking urges (mQSU)-brief and modified version of the Cigarette Evaluation Scale (mCES) across product type. mQSU-brief factor scores reflect the difference between ratings taken after product usage, minus ratings taken before product usage; mCES factor scores reflect ratings following product usage only. Error bars reflect the standard error of the mean.
Behavioral Results
Behavioral analyses were conducted for the same 22 participants included in the ERP analyses. Overall target accuracy for the oddball task was near perfect (M = 99.19%, SE = .12%), with a mean response time of 372.57ms (SE = 8.59ms). No significant changes in accuracy (P > .567) or response time (P > .362) following product usage were identified as a function of product type.
ERP Results
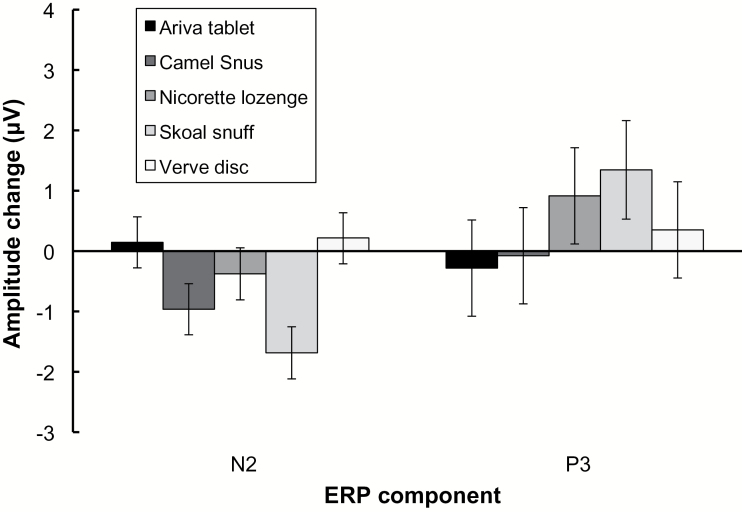
ERP analyses were conducted on a subset of 22 participants, for which complete data was available (see Figure 2 and Supplementary Figure). Analysis of N2 difference scores revealed a significant effect of product type (F (4,97) = 3.70, P = .008). Follow-up paired comparisons revealed that Skoal elicited a significantly larger N2 relative to Verve (corrected P = .017) and Ariva (corrected P = .023). Analysis of P3b difference scores revealed no significant effect of product type (P = .609).
Figure 2.
Event-related potential (ERP) component difference scores across product type. Difference scores reflect component amplitude following product usage, minus component amplitude prior to product usage. Error bars reflect the standard error of the mean. See Supplementary Figure for traditional ERP plots.
EEG Results
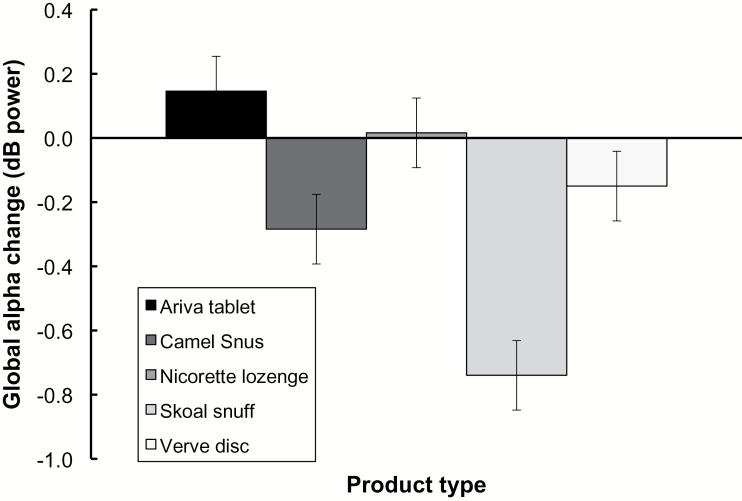
EEG analyses were conducted for a subset of 23 participants, for which complete data was available (see Figure 3). Analysis of global alpha difference scores revealed a significant main effect of product type (P = .0001). However, neither the main effect of time (P = .169), nor the product by time interaction (P = .914) was significant. Follow-up paired comparisons revealed that Skoal exhibited enhanced alpha suppression relative to Ariva (corrected P = .001), Snus (corrected P = .007), Verve (corrected P = .001), and Nicorette (corrected P = .001). Additionally, Snus exhibited enhanced alpha suppression relative to Ariva (corrected P = .014).
Figure 3.
Global alpha power difference scores as a function of product type. Difference scores reflect global alpha power amplitude following product usage (collapsed across time point), minus global alpha power prior to product usage. Error bars reflect the standard error of the mean.
Relationship Between Electrophysiology and Subjective Scales
Of the subjective scales analyzed in the present report, N2 difference scores only correlated negatively with the mQSU-brief “desire to smoke” factor (r = −0.22, n = 114, P = .015), whereas the P3b difference scores did not correlate with any of the subjective scales (all P > .186). Global alpha suppression was found to correlate negatively with the mQSU-brief “relief from withdrawal” factor (r = −0.25, n = 114, P = .007). Additionally, global alpha suppression was found to correlate positively with the “psychological reward” (r = 0.33, n = 114, P = .0004) and “satisfaction” (r = 0.25, n = 114, P = .008) mCES factors. Please note that in order to improve the interpretability of these correlations, the sign of the N2 and alpha suppression difference scores were reversed. See Table 1 for a list of correlation values.
Table 1.
Correlations Between Electrophysiology and Subjective Measures
| mQSU-brief factors | mCES factors | ||||
|---|---|---|---|---|---|
| Relief from withdrawal | Intention to use smokeless tobacco | Psychological reward | Satisfaction | Aversion | |
| N2 | −0.09 | −0.22* | 0.12 | 0.07 | −0.04 |
| P3b | 0.01 | 0.02 | −0.01 | 0.13 | 0.04 |
| Alpha | −0.25** | −0.10 | 0.33*** | 0.25** | 0.14 |
mQSU = modified version of the Questionnaire on Smoking urges; mCES = modified version of the Cigarette Evaluation Scale. mQSU-brief factors reflect the difference score of post minus pre product usage. To improve the interpretability the correlations, the sign of the N2 and alpha suppression difference scores were reversed.
*P < .05, **P < .01, ***P < .001.
Discussion
The present investigation was aimed at exploring the combined use of electrophysiology and subjective measures as a tool to assess the consumer acceptability of smokeless tobacco products. Questionnaires assessing product satisfaction and elicited psychological reward showed a general preference for Skoal (the product with the highest nicotine content) over the other four products tested (Ariva, Snus, Verve, Nicorrete lozenge), however, many of these comparisons were not statistically significant after multiple comparisons correction. In contrast, the electrophysiological measures of attention (N2) and arousal (alpha suppression) were most pronounced for Skoal. Importantly, alpha suppression correlated with measures of product satisfaction and psychological reward, not aversion, supporting the notion that one of the primary reasons that tobacco products are used (and liked) is due to their ability to influence executive function.8–11 The current results reinforce the importance of assessing electrophysiological measures of executive function when testing the consumer acceptability of novel tobacco products.
A traditional analysis of consumer acceptability using subjective measures revealed no significant differences across products for reducing smoking urges (mQSU-brief) or satisfaction (mCES). The fact that withdrawal reduction did not significantly differ as a function of product, despite substantial differences in nicotine content across products, is likely the result of the low-dependence level of the sample studied (mFTND = 1.27). The low dependency level of the current sample and lack of withdrawal effects makes the product liking and executive function changes particularly fascinating in the current study. Assessment of the mCES “psychological reward” factor demonstrated that reward ratings of Skoal were significantly higher than those for Verve. The only subjective scale that revealed more than one significant difference between products was the mCES “aversion” factor, with Skoal being rated as more aversive than all other products. Interpretation of the subjective results alone might suggest that consumers’ responses to the products were largely undifferentiated, with the exception of perceived aversion. Given previous work demonstrating that satisfaction, not aversion or psychological reward, is predictive of real-world product usage,5 the subjective results would lead to no firm predictions of actual product use. However, a more substantial problem is that the subjective results alone may overlook other neuropsychological aspects of consumer acceptability, such as the ability of products to influence executive function. When the electrophysiological results are analyzed, there appears to be a considerable implicit positive bias toward Skoal in terms of its ability to modulate attention/arousal (measured by N2 increases and alpha suppression), suggesting that neurophysiological measures are useful to gain a more comprehensive understanding of product acceptability.
Comparison of alpha suppression revealed robust differences between products; Skoal elicited substantial alpha suppression compared to all four other products and Snus elicited enhanced alpha suppression compared to Ariva. Alpha band power has previously been linked to cortical arousal,16,17 with alpha suppression associated with increased frontal/parietal cortex activity.36 Additionally, one of the most consistent electrophysiological changes following tobacco use is an alpha band activity change, reflecting either global alpha suppression,19,21,20 or selective suppression of low alpha (8–10 Hz)18 and/or increases in high alpha (12–14 Hz).37–39 It should be noted that while the current investigation is not the first to identify alpha suppression across the 8–12 Hz alpha band in response to tobacco product use,19,21,20 recently, it has been more common to identify selective suppression of low alpha (8–10 Hz)18 and/or increases in high alpha (12–14 Hz).37–39 Given that peak alpha frequency is known to vary across individuals,40 the subtle differences in terms of the alpha effects observed across studies may be due to differences in the average peak alpha frequency of the participants included in a given sample. Additionally, differences in the present report may be due to the fact that our investigation employed an eyes-open resting state methodology. Future research should further investigate potential differences in tobacco-driven alpha effects as a function of individual differences or recording-session methodology (eyes-open vs. eyes-closed). Nonetheless, given that a primary reason tobacco products are used is to increase arousal,8–11 the enhanced alpha suppression for Skoal may relate to an implicit preference for the Skoal product not captured by traditional subjective measures. This preference for Skoal is to be expected, given that Skoal had the highest nicotine content.
It is important to note that while Skoal was rated as both highly aversive and yielded enhanced alpha suppression, alpha suppression was uncorrelated with aversion. Instead, alpha suppression was positively correlated with satisfaction and psychological reward (and correlated negatively with smoking urges). The positive correlation between alpha suppression and satisfaction or psychological reward fits with previous work demonstrating that a primary reason tobacco products are used is to improve arousal levels.8–11 Although subjective ratings of Skoal, in terms of satisfaction or psychological reward, did not reach statistical significance for most comparisons, the alpha suppression measure was sensitive to implicit differences among products. Given the link between arousal and product use, this suggests that repeated exposure to Skoal may actually lead to product adoption and ultimately abuse. This finding highlights the potential utility of using direct neurophysiological recordings as a more objective measure to compliment traditional subjective assessment of consumer acceptability.
In addition to investigating changes in cortical arousal (alpha suppression), the present study analyzed how each product influenced task-related attention with the N2 and P3b components. Previous work has shown that the P3b is enhanced following nicotine and tobacco use.23,24 Although the P3b was not significantly influenced by product type, this may reflect the relatively smaller sample size of the present investigation. Previous work has also generally demonstrated modulation of the N2 in response to nicotine and tobacco use, although the results have been mixed (for a review see: Pritchard et al.22). In the present report, N2 was larger for Skoal compared to Ariva/Verve. While P3b is influenced by attentional enhancement, it is also sensitive to higher-level factors.13,14 In contrast, N2 might relate more directly to sensory attention. Specifically, the lateral-occipital topography of N2 suggests this component may reflect a similar process as that previously described by the selection negativity,41 with N2 indexing enhanced top-down control over sensory cortex. Therefore, selective enhancement of N2 for Skoal (compared to Ariva/Verve) suggests that Skoal elicits up-regulation of attentional control. Given that a primary reason for tobacco product use is improving attention,8–11 participants may be more likely to use Skoal for this purpose.
It is important to note that the product that elicited the greatest changes in alpha suppression and N2 enhancement, was the highest nicotine-containing product. This is consistent with literature indicating that the ability of tobacco products to improve executive functioning is driven by nicotine, as compared to placebo.18,23,42,43 This literature would suggest that the smokeless tobacco product that causes the greatest influence on executive function does so as the result of its nicotine content alone. However, it is also known that factors other than nicotine can influence the response to a product44–46 and not all studies of nicotine have found evidence for nicotine alone influencing executive function.47 Therefore, it is also possible that factors unrelated to nicotine content contribute to these results and further work delineating exactly what aspects of a tobacco product drive changes in executive function are needed. Nonetheless, the present report demonstrates the importance of measuring executive function when assessing the consumer acceptability of tobacco products.
Taken together, the assessment of subjective measures of consumer acceptability, as well as objective measures of arousal and attention, provide a comprehensive description of consumers’ response to the products tested. While subjective measures have long been used to assess consumer acceptability of tobacco products, the current results suggest that the conclusions drawn from subjective measures alone may be limited or lack sensitivity in certain situations. Electrophysiological measures such as alpha suppression and N2 enhancement detected differences across products missed by traditional subjective measures. Additionally, electrophysiological measures have the added benefit of being directly tied to underlying neurocognitive processes and may be less influenced by conscious biases.
Instead of supplanting current subjective measures, electrophysiology demonstrates promise as a complimentary tool in the assessment of consumer acceptability. Previous work has suggested that consumer acceptability is a multidimensional construct composed of perceived aversion, satisfaction, and psychological reward derived from a product.7 However, tobacco products are also used for their ability to improve cognitive function,8–11 and such changes are readily detectable using electrophysiological measures. The present results suggest the need to revise the current understanding of how consumer acceptability is conceptualized and assessed. We propose that consumer acceptability arises not only from multiple psychological factors, readily assessed using traditional subjective measures, but also cognitive changes that may be better captured through neurophysiological assays. Therefore, future work assessing consumer acceptability should also employ a multidimensional/multi-method approach to studying consumer acceptability, when possible.
Supplementary Material
Supplementary Figure can be found online at http://www.ntr.oxfordjournals.org
Funding
Research reported in this publication was supported by grant number 5R21DA030622 from NIH/National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH/NIDA.
Declaration of Interests
None declared.
Supplementary Material
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. www.surgeongeneral.gov/library/reports/50-years-of-progress/. Accessed February 1, 2015. [Google Scholar]
- 2. Rees VW, Kreslake JM, Cummings KM, et al. Assessing consumer responses to PREPs: a review of tobacco industry and independent research methods. Cancer Epidemiol Biomark Prev. 2009;18(12):3225–3240. doi:10.1158/1055-9965.EPI-09-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caldwell B, Burgess C, Crane J. Randomized crossover trial of the acceptability of snus, nicotine gum, and Zonnic therapy for smoking reduction in heavy smokers. Nicotine Tob Res. 2010;12(2):179–183. doi:10.1093/ntr/ntp189. [DOI] [PubMed] [Google Scholar]
- 4. Hatsukami DK, Jensen J, Anderson A, et al. Oral tobacco products: preference and effects among smokers. Drug Alcohol Depend. 2011;118(2–3):230–236. doi:10.1016/j.drugalcdep.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatsukami DK, Zhang Y, O’Connor RJ, Severson HH. Subjective responses to oral tobacco products: scale validation. Nicotine Tob Res. 2013;15(7):1259–1264. doi:10.1093/ntr/nts265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westman EC, Levin ED, Rose JE. Smoking while wearing the nicotine patch-is smoking satisfying or harmful. Clin Res. 1992;40:A871–A871. [Google Scholar]
- 7. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi:10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- 8. Russell MAH, Peto J, Patel UA. The classification of smoking by factorial structure of motives. J R Stat Soc Ser Gen. 1974;137(3):313–346. doi:10.2307/2344953. [Google Scholar]
- 9. Pomerleau OF, Pomerleau CS. Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation. Neurosci Biobehav Rev. 1984;8(4):503–513. doi:10.1016/0149-7634(84)90007-1. [DOI] [PubMed] [Google Scholar]
- 10. Cook BL, Wayne GF, Keithly L, Connolly G. One size does not fit all: how the tobacco industry has altered cigarette design to target consumer groups with specific psychological and psychosocial needs. Addiction. 2003;98(11):1547–1561. doi:10.1046/j.1360-0443.2003.00563.x. [DOI] [PubMed] [Google Scholar]
- 11. Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing: nicotine and attention. Addict Biol. 2009;14(1):32–42. doi:10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- 12. Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2007;118(10):2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131(4):510–532. doi:10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- 15. O’Connell RG, Dockree PM, Kelly SP. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat Neurosci. 2012;15(12):1729–1735. doi:10.1038/nn.3248. [DOI] [PubMed] [Google Scholar]
- 16. Barry RJ, Clarke AR, Johnstone SJ, Brown CR. EEG differences in children between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2009;120(10):1806–1811. doi:10.1016/j.clinph.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 17. Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2007;118(12):2765–2773. doi:10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 18. Evans DE, Sutton SK, Oliver JA, Drobes DJ. Cortical activity differs during nicotine deprivation versus satiation in heavy smokers. Psychopharmacology (Berl). 2014;232(11):1879–1885. doi:10.1007/s00213-014-3821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herning RI, Jones RT, Bachman J. EEG changes during tobacco withdrawal. Psychophysiology. 1983;20(5):507–512. doi:10.1111/j.1469-8986.1983.tb03004.x. [DOI] [PubMed] [Google Scholar]
- 20. Pritchard WS. Electroencephalographic effects of cigarette smoking. Psychopharmacology (Berl). 1991;104(4):485–490. doi:10.1007/BF02245654. [DOI] [PubMed] [Google Scholar]
- 21. Philips C. The EEG changes associated with smoking. Psychophysiology. 1971;8(1):64–74. doi:10.1111/j.1469–8986.1971.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 22. Pritchard WS, Sokhadze E, Houlihan M. Effects of nicotine and smoking on event-related potentials: a review. Nicotine Tob Res. 2004;6(6):961–984. doi:10.1080114622200412331324848. [DOI] [PubMed] [Google Scholar]
- 23. Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG, Drobes DJ. Nicotine deprivation influences P300 markers of cognitive control. Neuropsychopharmacology. 2013;38(12):2525–2531. doi:10.1038/npp.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knott V, Choueiry J, Dort H, et al. Baseline-dependent modulating effects of nicotine on voluntary and involuntary attention measured with brain event-related P3 potentials. Pharmacol Biochem Behav. 2014;122:107–117. doi:10.1016/j.pbb.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 25. Pickworth WB, Herning RI, Henningfield JE. Electroencephalographic effects of nicotine chewing gum in humans. Pharmacol Biochem Behav. 1986;25(4):879–882. doi:10.1016/0091-3057(86)90401–6. [DOI] [PubMed] [Google Scholar]
- 26. Knott VJ. Electroencephalographic characterization of cigarette smoking behavior. Alcohol. 2001;24(2):95–97. doi:10.1016/S0741-8329(00)00140-3. [DOI] [PubMed] [Google Scholar]
- 27. Knott VJ. Neurophysiological aspects of smoking behaviour: a neuroelectric perspective. Br J Addict. 1991;86(5):511–515. [DOI] [PubMed] [Google Scholar]
- 28. Edwards JA, Warburton DM. Smoking, nicotine and electrocortical activity. Pharmacol Ther. 1982;19(2):147–164. [DOI] [PubMed] [Google Scholar]
- 29. Domino EF. Effects of tobacco smoking on electroencephalographic, auditory evoked and event related potentials. Brain Cogn. 2003;53(1):66–74. [DOI] [PubMed] [Google Scholar]
- 30. Conrin J. The EEG effects of tobacco smoking–a review. Clin Electroencephalogr. 1980;11(4):180–187. [DOI] [PubMed] [Google Scholar]
- 31. Gartner CE, Hall WD, Chapman S, Freeman B. Should the health community promote smokeless tobacco (snus) as a harm reduction measure? PLoS Med. 2007;4(7):e185. doi:10.1371/journal.pmed.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. doi:10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8:213. doi:http://doi.org/10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 35. Debener S, Thorne J, Schneider TR, Campos Viola F. Using ICA for the analysis of mutli-channel EEG Data. In: Ullsperger M, Debener S, eds. Simultaneous EEG and fMRI: Recording, Analysis, and Application. Vol. 1. New York, NY: Oxford University Press; 2010:121–133. [Google Scholar]
- 36. Laufs H, Kleinschmidt A, Beyerle A, et al. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19(4):1463–1476. doi:10.1016/S1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 37. Domino EF, Shigeaki M. Effects of tobacco smoking on the topographic EEG I. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18(5):879–889. doi:10.1016/0278-5846(94)90104-X. [DOI] [PubMed] [Google Scholar]
- 38. Knott VJ, Harr A, Ilivitsky V, Mahoney C. The Cholinergic Basis of the Smoking-Induced EEG Activation Profile. Neuropsychobiology. 1998;38(2):97–107. doi:10.1159/000026524. [DOI] [PubMed] [Google Scholar]
- 39.Knott V, Kerr C, Hooper C, Lusk-Mikkelsen S. Variations in spontaneous brain electrical (EEG) topography related to cigarette smoking: acute smoking, drug comparisons, cholinergic transmission, individual differences and psychopathology. In: Domino E, ed. Brain Imaging of Nicotine and Tobacco Smoking. Ann Arbor, MI: NPP Books; 1995:167–189. [Google Scholar]
- 40. Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29(2–3):169–195. doi:10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 41. Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci. 1998;95(3):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Knott V, Shah D, Fisher D, et al. Nicotine and attention: event-related potential investigations in nonsmokers. Clin EEG Neurosci. 2009;40(1):11–20. doi:10.1177/155005940904000108. [DOI] [PubMed] [Google Scholar]
- 43. McClernon FJ, Froeliger B, Rose JE, et al. The effects of nicotine and non-nicotine smoking factors on working memory and associated brain function [published online ahead of print April 22, 2015]. Addict Biol. doi:10.1111/adb.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rose JE, Salley A, Behm FM, Bates JE, Westman EC. Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology (Berl). 2010;210(1):1–12. doi:10.1007/s00213-010-1810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl). 2005;184(3–4):274–285. doi:10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 46. Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77(4–5):683–687. doi:10.1016/S0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- 47. Cook MR, Gerkovich MM, Graham C, Hoffman SJ, Peterson RC. Effects of the nicotine patch on performance during the first week of smoking cessation. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2003;5(2):169–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.