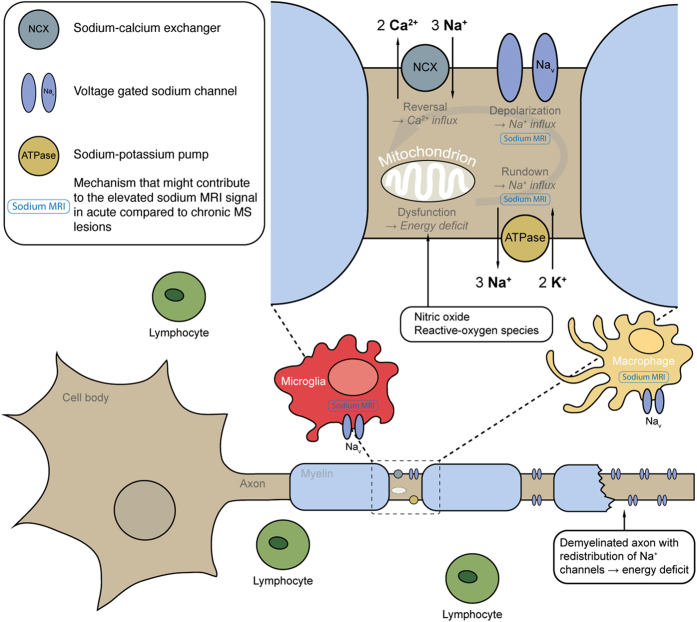
Figure 5. Overview of the pathological mechanisms in MS that might contribute to sodium MRI signal elevation in acute lesions.
The pathogenesis of MS involves a cascade of pathological events, including focal lymphocytic infiltration, microglia and macrophage activation, demyelination, and axonal degeneration2. Nav1.6 is the predominant sodium channel expressed in microglia and macrophages, and is up regulated upon activation of these cells in EAE and acute MS lesions35,36. Activated microglia and macrophages produce reactive-oxygen species4 and nitric oxide3 and thus induce mitochondrial dysfunction in neurons37,38 with subsequently reduced energy supply. Concurrently, re-distribution of Nav1.6 along demyelinated axons in EAE31 and acute MS lesions11 increases energy demand. The mismatch between reduced energy supply and elevated demand creates a virtual hypoxia39, which also impairs the sodium-potassium pump9, and leads to an intracellular accumulation of sodium ions12. This ionic imbalance promotes the sodium-calcium exchanger to operate in reverse13. Consecutive calcium import leads to injurious intracellular calcium levels with deleterious sequelae to the neuron9. Mechanisms of MS associated with intracellular sodium accumulation that possibly contribute to elevated average and fluid-attenuated sodium MRI signals are marked in blue. Also, hyper-cellularity itself might cause an increased fluid-attenuated signal. In fact, it may well be that both, intracellular sodium accumulation and hyper-cellularity contribute to the increased sodium signals observed in acute MS lesions as both are part of the same pathophysiologic mechanism.