Fig 3.
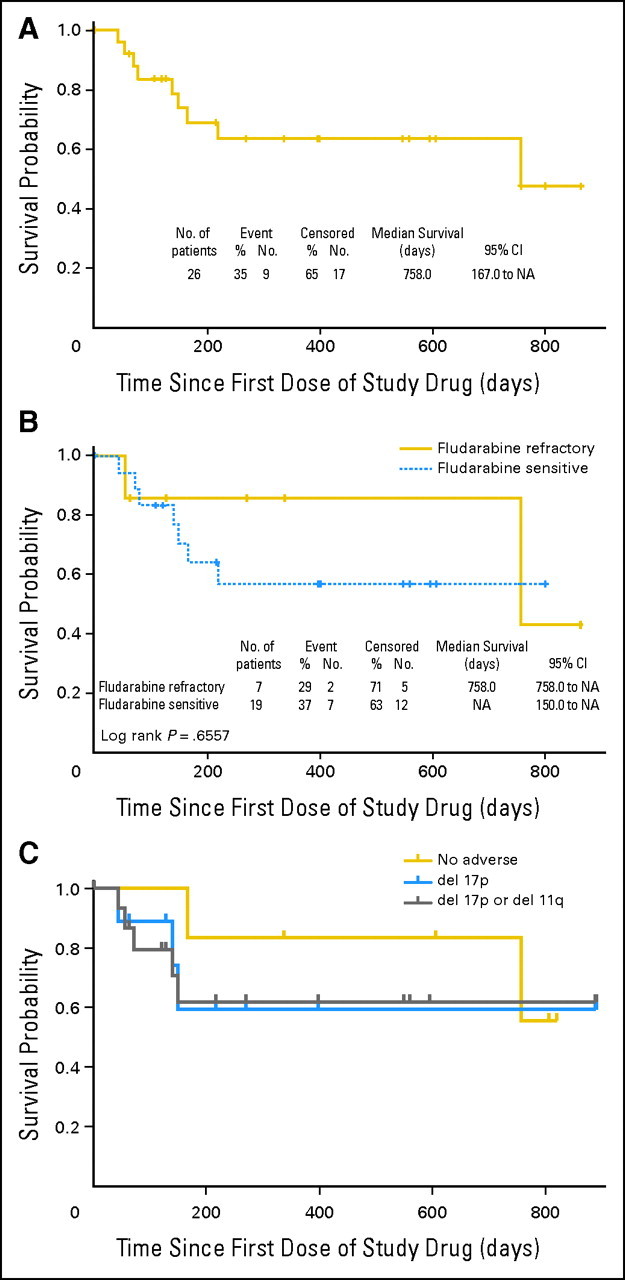
Durability of antileukemic activity of navitoclax. (A) The progression-free survival (PFS) for all patients receiving ≥ 110 mg/d navitoclax from study entry (n = 26) is displayed in a Kaplan-Meier plot. (B) PFS for a subset of these patients with fludarabine-refractory disease. Fluorescent in situ hybridization data were available for 22 of 26 patients receiving ≥ 110 mg/d navitoclax. (C) PFS for patients with del17p13.2 chronic lymphocytic leukemia (n = 9), either del17p13.2 or del11p22.3 (n = 16), or chronic lymphocytic leukemia with neither of these abnormalities (n = 6). NA, not applicable.
