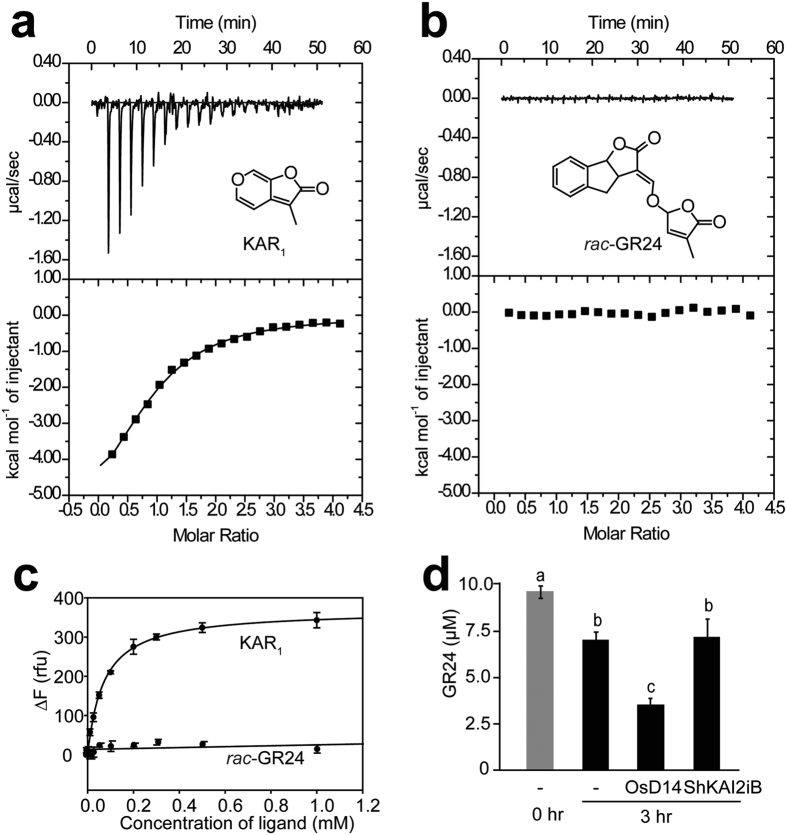
Figure 1. Binding specificity of ShKAI2iB.
(a,b) Results of ITC experiments of ShKAI2iB titrated with KAR1 (a) and rac-GR24 (b). Binding of KAR1 to ShKAI2iB exhibited a KD value of 77.6 ± 3.4 μM, along with ΔH (enthalpy change) of −6.48 ± 0.4 kcal mol–1, ΔS (entropy change) of –3.99 cal mol–1 deg–1 and N (number of sites) of 0.91 ± 0.05 (Supplementary Figure S4 and Table S2). (c) Changes of fluorescence intensity by the addition of KAR1 and rac-GR24 to ShKAI2iB. Intrinsic fluorescence was recorded at excitation wavelength of 285 nm and emission wavelength of 333 nm. Fitting using SigmaPlot 13.0 indicated that KD value was 70.0 ± 3.4 μM (n = 3) for KAR1 binding to ShKAI2iB. (d) Enzymatic degradation assay of rac-GR24. The data means ± SE of three independent experiments. Statistical differences between the groups were calculated with ANOVA analysis followed by Tukey–Kramer test. Bars with different letters are significantly different with p < 0.01.