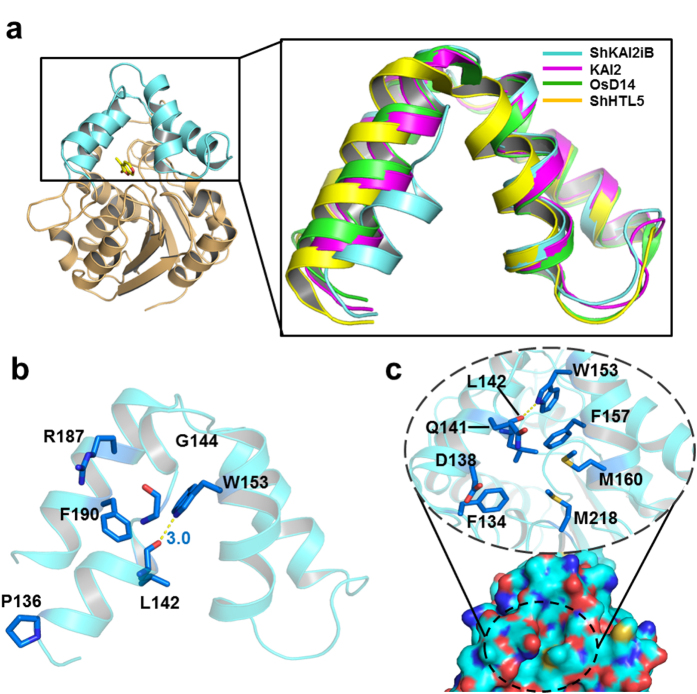
Figure 3. Structural basis for regulating the conformation of cap domain.
(a) Superposition of cap domain from ShKAI2iB in a ligand-free (apo) state (cyan) and KAI2 (apo, PDB ID 4JYP) (magenta), OsD14 (PDB ID 3WIO) (Green) and ShHTL5 (PDB ID 5CBK) (Yellow). (b) Regulatory residues in the cap domain of ShKAI2iB (ligand-free apo state). Blue sticks represent the residues involved in closed conformation of helix αD1. Hydrogen bond between Leu142 and Trp153 was indicated by yellow dashed line and bond length was shown in blue value (Å). (c) Surface representation (bottom) and residues around cavity entry of ShKAI2iB (top). Cyan, blue, red, and yellow surfaces represent carbon, nitrogen, oxygen, and sulfur atoms, respectively. In the dashed circle, some residues of helix αD1 and αD2, which are supposed to be the access to KAR1 binding pocket, are emphasized in close-up view. The entrance is closed and inaccessible to solvent.