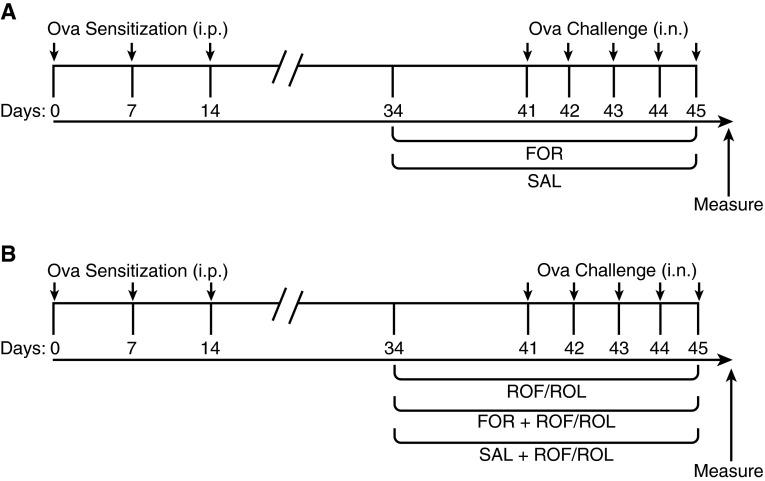
Figure 1.
Treatment protocol. Phenylethanolamine N-methyltransferase (PNMT)-knockout (KO) and wild-type (WT) mice received ovalbumin (Ova; 2 mg/kg/day in 2 mg of alum) intraperitoneally (i.p.), as indicated, on Days 0, 7, and 14, followed by intranasal (i.n.) challenge with Ova (1 mg/kg/day) or saline on Days 41–45 (arrows). (A) Groups of Ova sensitization and challenge (Ova S/C) PNMT-KO and WT mice received twice-daily formoterol (FOR; 5 μg/kg, intraperitoneally) or salmeterol (SAL; 1.5 μg/kg, intraperitoneally). (B) Groups of Ova S/C PNMT-KO and WT mice were administered roflumilast (ROF; 5 mg/kg) or rolipram (ROL; 5 mg/kg) alone via oral gavage, or groups were coadministered ROF (5 mg/kg) or ROL (5 mg/kg) twice daily with either FOR (5 μg/kg) or SAL (1.5 μg/kg). Duration of all drug administration was 12 days.