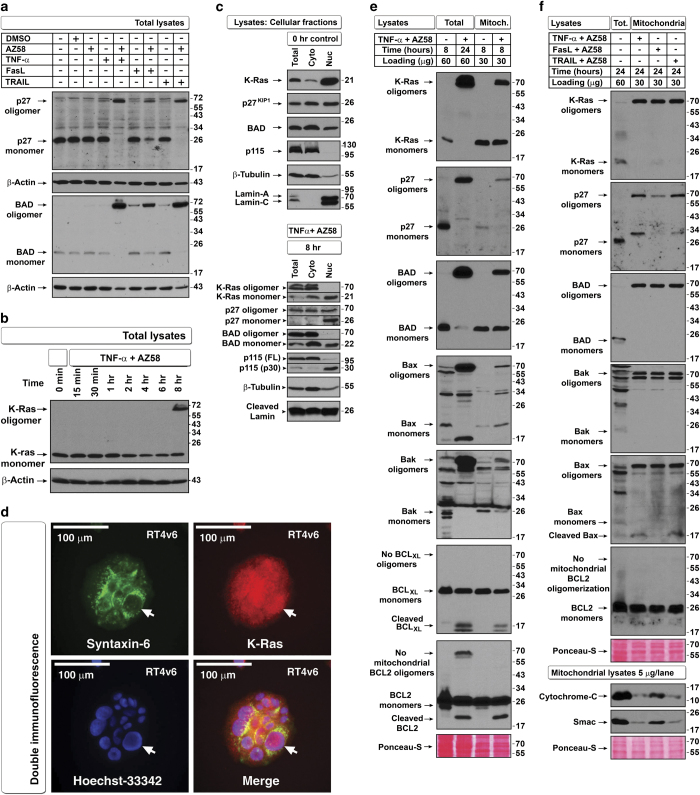
Figure 3.
Cleaved Bax but not oligomers of K-Ras, p27, BAD, Bax, and Bak in apoptotic mitochondria correlates with inhibition of transformation from blebbishields. (a) TNF-α and TRAIL induce MW shift in p27 and BAD more efficiently than FasL+AZ58 does at 24 h time point examined by western blotting. (b) TNF-α+AZ58 induces MW shift in K-Ras as early as 8 h after treatment. (c) Nuclear and cytoplasmic fractionation of control cells (top panel) and TNF-α+AZ58-treated apoptotic cells at 8 h (bottom panel) to explore localization of K-Ras, p27, and BAD. No full-length lamin A/C was detected in treated apoptotic cells. (Note that cleaved 30-kDa fragment of p115 can enter the nucleus during apoptosis.34). (d) Double immunofluorescence confirmation of nuclear localization of K-Ras (arrows). Syntaxin-6 marks perinuclear Golgi membranes. (e) Localization of oligomers in mitochondrial fractions as early as 8 h after TNF-α+AZ58 treatment. Note that neither BCLXL nor BCL2 from antiapoptotic members formed oligomers in mitochondria, although BCL2 oligomerized at non-mitochondrial sites. (f) Comparative mitochondrial oligomerization of K-Ras, p27, BAD, Bax, and Bak at 24 h and associated Smac and cytochrome-C release from mitochondria. FasL+AZ58 had reduced release of Smac and Cytochrome-C. First lane is total lysates except Smac and Cytochrome-C, which are control mitochondrial lysates.