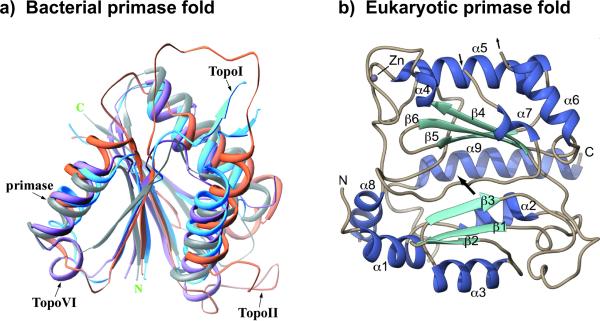
Figure 5.
Bacterial and eukaryotic primases adopt distinct chain folding patterns. (a) The structure of bacterial primases resembles the structure of topoisomerases, called a “Toprim” domain, and thus is thought to have shared a common ancestor with topoiosomerases. The figure shows a superimposition that includes the active site of E. coli primase, E. coli TopoI, S. cerevisiae TopoII and M. jannaschii TopoVI. Reprinted with permission of Elsevier from Figure 3(A) in Podobnik et al. (2000). (b) Human primase catalytic subunit adopts a folding pattern unrelated to topoisomerases, and instead has homology to the X-family of DNA polymerases. Figure adapted with permission from Figure 2 of Kilkenny et al. (2013) (see color version of this figure at www.informahealthcare.com/bmg).