Scheme 4.
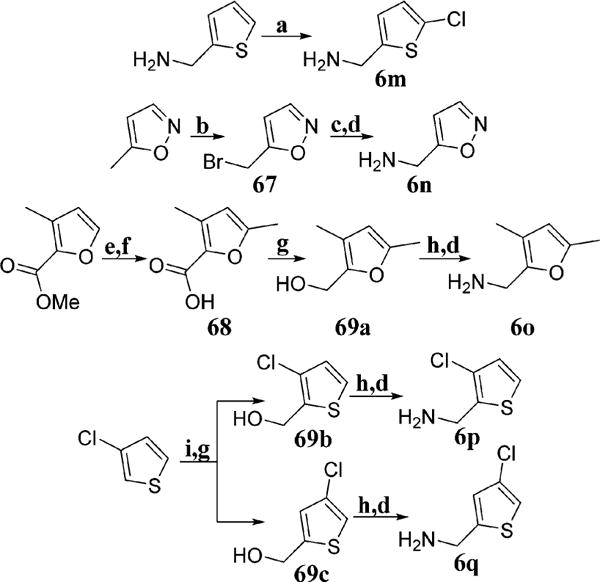
aConditions: (a) SO2Cl2, 15 °C, AcOH:Et2O (9:1), 1 h; (b) NBS, AIBN, CCl4, 80 °C, 4 h; (c) NaN3, MeOH:H2O (10:1), 25 °C, 12 h; (d) PPh3, THF:H2O (10:1), 25 °C, 12 h; (e) LiOH, THF:MeOH:-H2O (9:1:1), 25 °C, 10 h; (f) LDA, MeI, THF, −40 °C, 3 h; (g) LiAlH4, THF, 0–25 °C, 12 h; (h) DBU, DPPA, toluene, 25 °C, 12 h; (i) n-BuLi, CO2(g), THF, −78 °C, 2 h.
