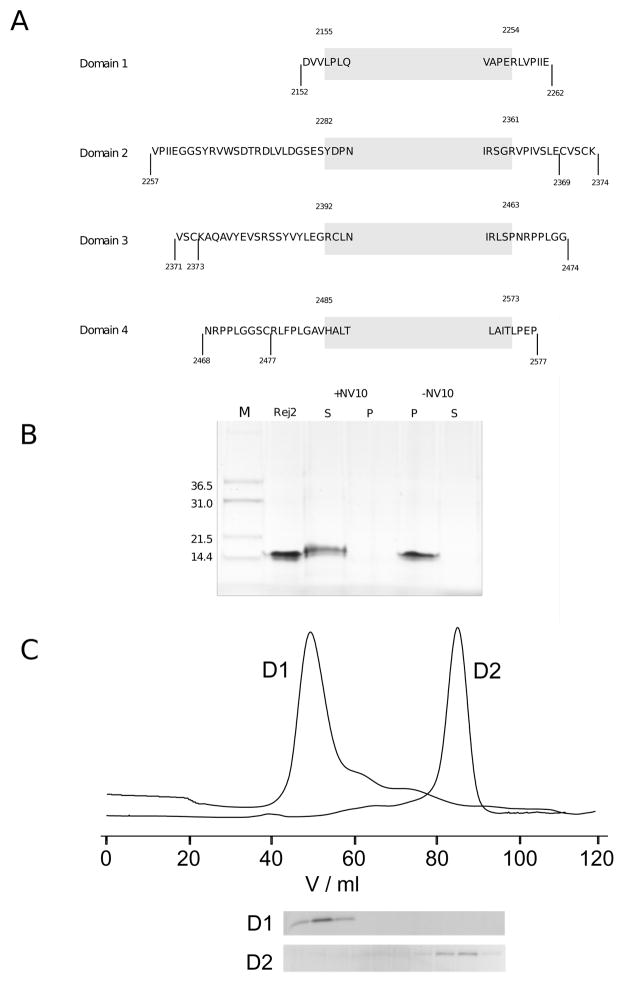
Figure 3.
A: Overview of expression constructs. Shown are for all domains the various constructs that were created for expression trials in bacteria. The shaded box indicates the extent of the domain definition which we here take to start two amino acids before the first residue of the first β-strand and to end two residues after the last amino acid of the last β-strand as shown in Fig. 2. A few amino acids are shown at the start and the end of the box to help orientation. Expression was tested for each domain with two constructs: one as indicated by the shaded box, the other indicated by the markers at the end points. The only variation exists for domain 2 where the new, intermediate length construct is indicated that is expressed solubly. B: Refolding of domain 2. Shown is the purified protein before and after refolding in refolding buffer with and withouth NV10 polymer. Soluble (S) and insoluble (P) fractions are shown separated. C: Preparative gel filtration purification of domains 1 and 2 expressed soluble. Elution fractions of nickel affinity purifications of both domains were loaded on a Superdex 75 16/60 preparative column. Domain 2 emerges roughly in agreement with being a monomer while domain 1 appears close to the exclusion volume suggesting a heavily aggregated yet well soluble state.