FIGURE 3.
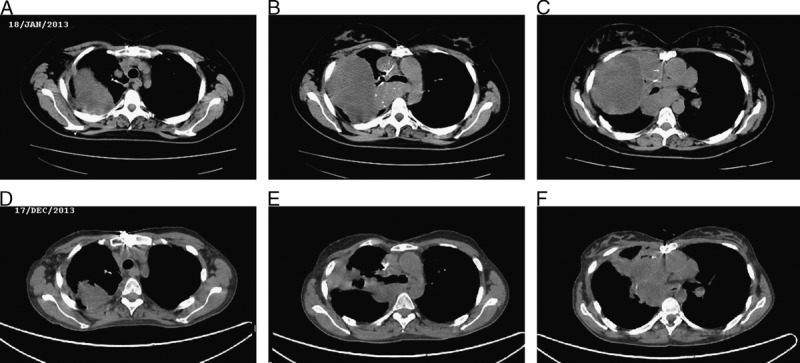
Objective partial response based on RECIST criteria (reduction of >50%) was observed after 2 courses of Lutathera. Figure 3 shows baseline thoracic computed tomography scan (A–C) and after 11 months (D–F). The overall duration of her response (stabilization with cold lanreotide and partial response with Lutathera) exceeded 1 year. The objective reduction made possible a surgical approach in an attempt to reduce the tumor load, and therefore a thoracotomy and debulking was carried out in January 2014. Unfortunately, an accidental rupture of the right atrium with profuse bleeding occurred during surgery. She died in April 2014 after a long stay in the intensive care unit. Lutathera is an investigational medical product in late-stage development.
