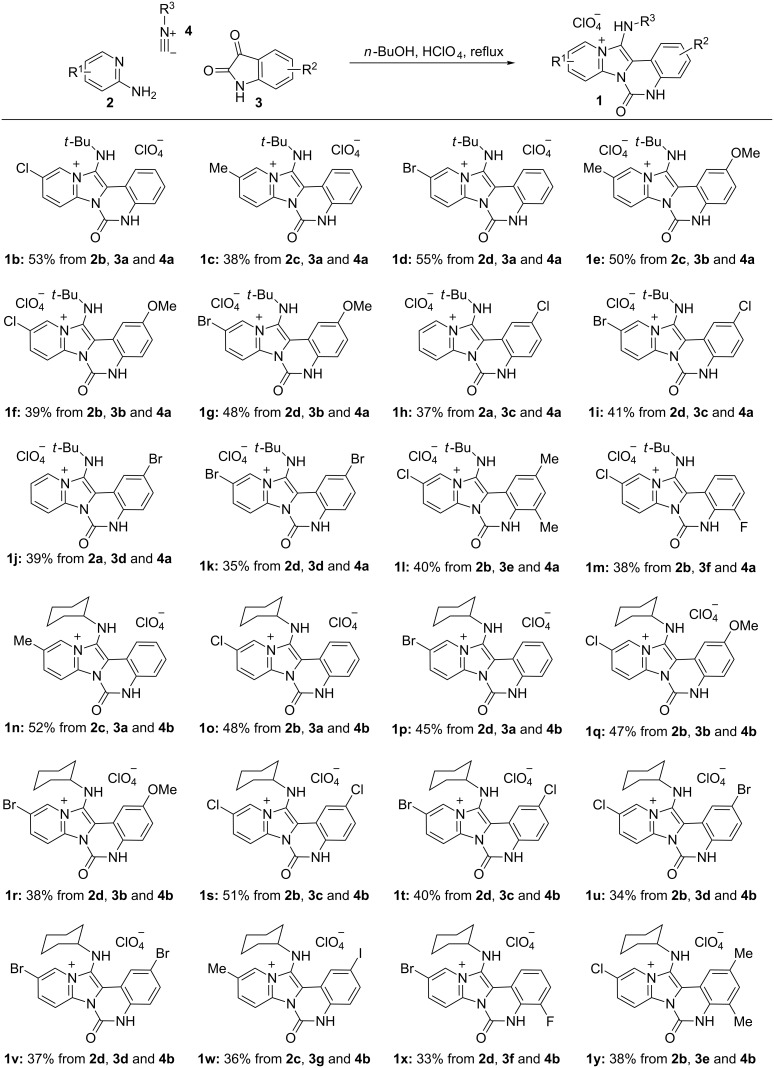
Figure 1.
Syntheses of imidazo[1,2-a]pyridine derivatives. Reaction conditions: 2 (1.35 mmol), 3 (1 mmol), 4 (1.35 mmol), HClO4 (1 mmol), n-BuOH (4 mL), reflux. Yields refer to isolated yields. 2b R1 = 4-Cl; 2c R1 = 4-Me, 2d R1 = 4-Br; 3b R2 = 5-OMe; 3c R2 = 5-Cl; 3d R2 = 5-Br; 3e R2 = 5,7-Me2; 3f R2 = 7-F; 3g R2 = 5-I; 4b R3 = cyclohexyl.