Table 1.
Synthesis of imidazo[1,2-a]pyridine derivatives in indicated conditions [36].a
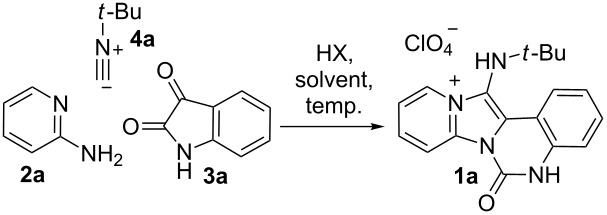 | ||||
| Entry | Solvent | Acid | Temperature | Yield (%) |
| 1 | MeOH | HClO4 | rt | 4 |
| 2 | MeOH | HClO4 | reflux | 11 |
| 3 | MeCN | HClO4 | reflux | –b |
| 4 | DMF | HClO4 | 100 °C | 10 |
| 5 | EtOH | HClO4 | reflux | 17 |
| 6 | iPrOH | HClO4 | reflux | 22 |
| 7 | n-BuOH | HClO4 | reflux | 30 |
| 8 | iBuOH | HClO4 | reflux | 21 |
| 9 | t-BuOH | HClO4 | reflux | 19 |
| 10 | isopentyl alcohol | HClO4 | reflux | 17 |
| 11 | CF3CH2OH | HClO4 | reflux | 13 |
| 12 | n-BuOH | PTSA | reflux | 20 |
| 13 | n-BuOH | HCl | reflux | – |
| 14 | n-BuOH | AcOH | reflux | – |
| 15 | n-BuOH | HClO4 | reflux | 42c |
aConditions: 2a (1 mmol), 3a (1 mmol), 4a (1mmol), and acid HX (1 mmol) in 4 mL of solvent; b"–" indicates that the product was not obtained; cconditions: 2a (1.35 mmol), 3a (1 mmol), 4a (1.35 mmol), and acid HX (1 mmol) in 4 mL of solvent.
