Table 1.
Rh-catalyzed Mannich-type reaction using various α,β-unsaturated esters.
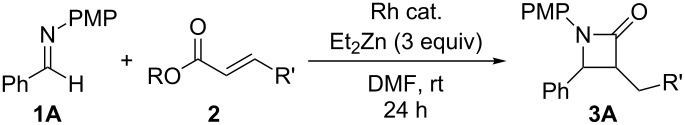 | ||||
| Entry | Substrate 2 | Rh cat. (mol %) | Product | Yield (%)a |
| 1 |
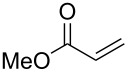 2a |
RhCl(PPh3)3 (2) |
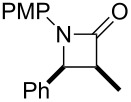 3Aa |
88 [syn/anti = 96:4]b |
| 2 | [RhCl(cod)]2 (1) | 78 [syn/anti = 88:12]b |
||
| 3 |
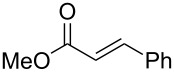 2b |
RhCl(PPh3)3 (4) |
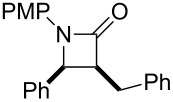 3Ab |
nd |
| 4 | [RhCl(cod)]2 (2) | 66c | ||
| 5 |
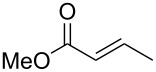 2d |
RhCl(PPh3)3 (2) |
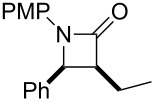 3Ad |
34c |
| 6 | [RhCl(cod)]2 (2) | 77c | ||
| 7 |
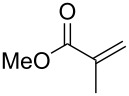 2e |
[RhCl(cod)]2 (2) |
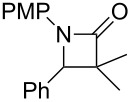 3Ae |
98 |
| 8 |
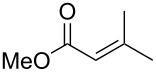 2f |
[RhCl(cod)]2 (2) |
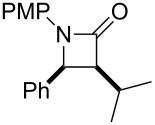 3Af |
nd |
| 9 |
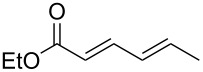 2g |
[RhCl(cod)]2 (2) |
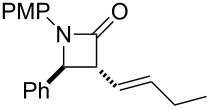 3Ag |
59d [E/Z = 93:7]e |
| 10 |
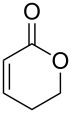 2h |
RhCl(PPh3)3 (2) |
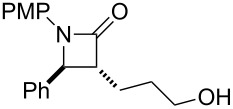 3Ah |
11d |
| 11 | [RhCl(cod)]2 (2) | 20d | ||
| 12 |
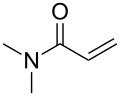 2i |
[RhCl(cod)]2 (2) |
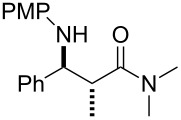 4Ai |
27 [syn/anti = 18:82]b |
aIsolated yield. bDiastereomeric ratio [syn/anti] after purification. cThe syn product was obtained as the sole product. dThe anti product was obtained as the sole product. eE/Z ratio by 1H NMR.
