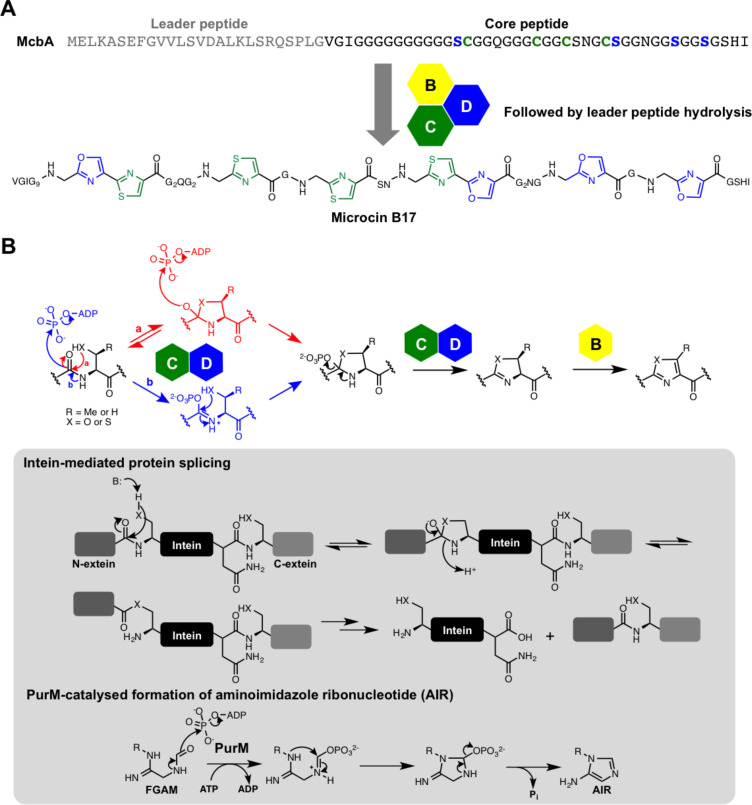
Figure 3.
Formation of thiazoles and oxazoles in RiPPs. A) Biosynthesis of microcin B17. B) Mechanistic models for the introduction of azol(in)es into microcin, where pathway a was reported by the authors as the likely order of steps. An analogous mechanism was proposed in the biosynthesis of trunkamide, but with the transfer of AMP instead of phosphate. Inset: partial mechanism of intein-mediated protein splicing, which proceeds via a reversible hemiorthoamide, and the proposed mechanism of PurM-catalysed conversion of formylglycinamide ribonucleotide (FGAM) into aminoimidazole ribonucleotide (AIR), which involves activation of an amide by ATP and a 5-endo-trig cyclisation.