Table 18.
Examples of the Schenck rearrangement.
| Entry | Allylic isomer A | Allylic isomer B | Ref. |
| 1 |
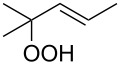 404a |
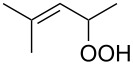 404b |
[424] |
| At 40 °C in non-polar solvents, an approximately equimolar mixture of A and B is formed | |||
| 2 |
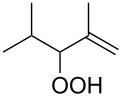 405a |
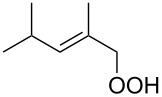 405b |
[414] |
| In hexane, A is rearranged to an equilibrium mixture of ~80% A and ~20% B | |||
| 3 |
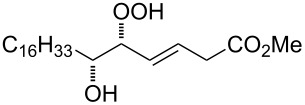 406a |
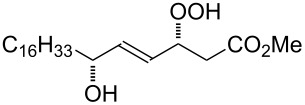 406b |
[425] |
| At 60–70 °C in C6H6 or MeCN in the presence of TBHN or AIBN within 16–22 h, a 50:50 A:B mixture is formed | |||
| 4 |
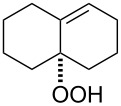 407a |
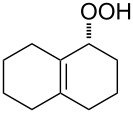 407b |
[426] |
| In CCl4 at 40 °C for 141 h, the rearrangement proceeds by 80% | |||
| 5 |
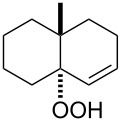 408a |
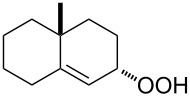 408b |
[427] |
| In CDCl3, the rearrangement of A into B is completed in 24 h | |||
| 6 |
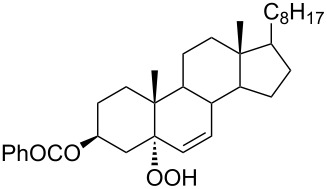 409a |
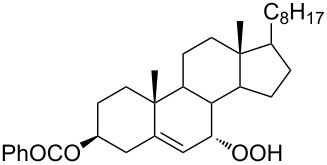 409b |
[428] |
| In CDCl3, the rearrangement is completed in 72 h | |||
| 7 |
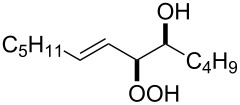 410a |
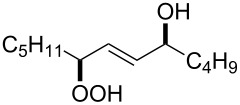 410b |
[429] |
| In C6H6 in presence of 10 equiv TBHP and 20 mol % DTBN at 40 °C for 16 h, isomers A and B are formed in equal amounts | |||
| 8 |
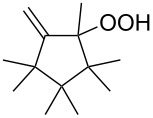 411a |
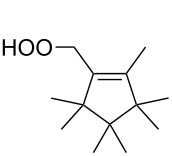 411b |
[430] |
| In CDCl3 the rearrangement is completed in 48 h | |||
| 9 |
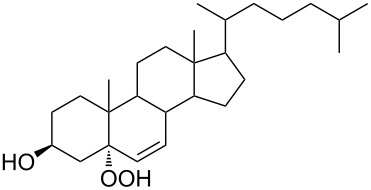 412a |
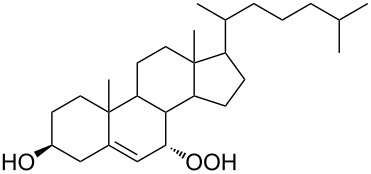 412b |
[431] |
| In CHCl3 for 5 d at room temperature, only partial conversion | |||
| 10 |
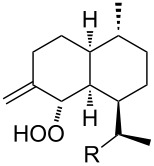 413a |
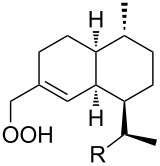 413b |
[432] |
| In CDCl3 the rearrangement is completed after 3–4 weeks; R: CO2H, CO2Me, CH2OH, CH3 | |||
| 11 |
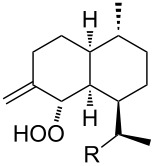 414a |
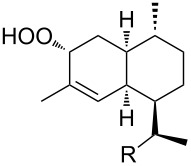 414b |
|
| In CDCl3 the rearrangement is completed after 2–4 weeks; R: CO2H, CO2Me, CH2OH, CH3 | |||
| 12 |
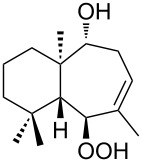 415a |
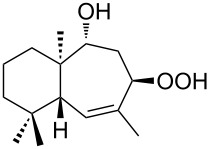 415b |
[433] |
| In CDCl3 the rearrangement is completed after 2 d | |||
| 13 |
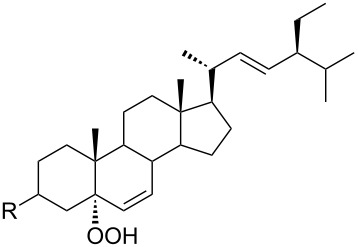 416a |
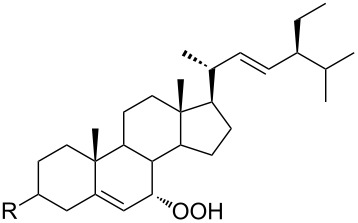 416b |
[434] |
| In pyridine for 24 h, R: OH,CH3COO, F, Cl, conversion 12–58% | |||
| 14 |
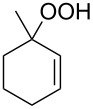 417a |
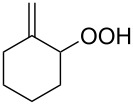 417b |
[435] |
| In a 5 M solution of LiClO4 in Et2O the rearrangement is completed in 24 h | |||
| 15 |
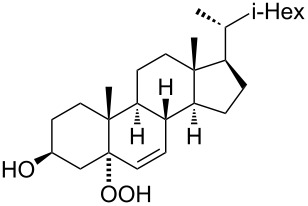 418a |
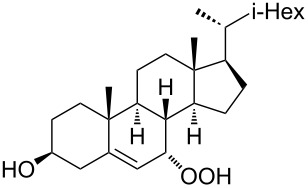 418b |
[436] |
| In CDCl3/D2O, lyophilized PBS buffer at pH 7 for 20 h, the conversion is 14% | |||
| 16 |
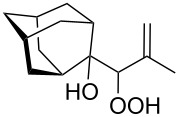 419a |
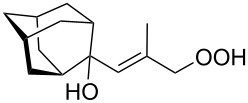 419b |
[136] |
| In CH2Cl2 at −78 °C with BF3·OEt2 (1 mol %) | |||
| 17 |
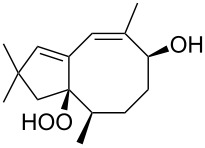 420a |
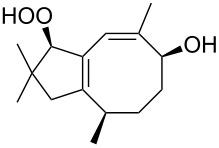 420b |
[437] |
| In MeCN/H2O, only partial conversion. | |||
