Table 23.
Base-catalyzed intramolecular rearrangement of 1,2-dioxetanes.
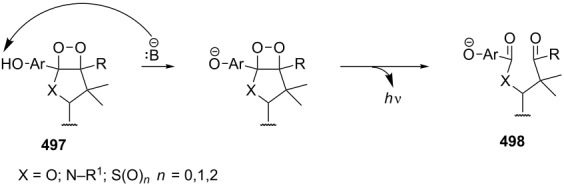 | |||||
| Entry | X | R | Ar-OH | Reaction conditions | Ref. |
| 1 | O | t-Bu |
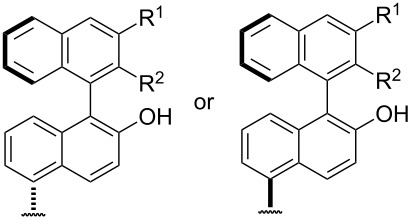 R1, R2 = H, OMe, CO2Me, CO2H, CH2OH |
TBAF in DMSO at 25 °C for 1 h | [463] |
| 2 | O | t-Bu |
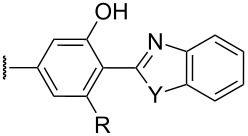 R = H, OMe; Y = O, S |
NaOH in СH3CN/H2O at 45 °C | [464] |
| 3 | O | t-Bu | 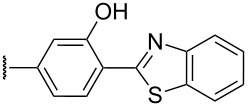 |
TBAF in DMSO (NMP or DMF) at 45–100 °C | [465] |
| 4 | O | Me, Et, iPr, iBu | in NMP at 50–100 °C or in TBAF/NMP at 35–60 °C | [466] | |
| 5 | O | t-Bu | 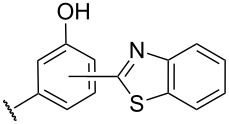 |
TBAF in CH3CN at 45 °C | [467] |
| 6 | NBoc | t-Bu | 3-OH-C6H4 3-OMe-C6H4 6-OH-C10H6 |
TBAF in DMSO at 25 °C | [468–469,473] |
| 7 | S, SO, S(O)2 | t-Bu | 3-OH-C6H4 3-OMe-C6H4 3-OAc-C6H4 |
TBAF in DMSO at 25 °C | [472] |
| 8 | O | t-Bu | HO-phenanthrenyl | TBAF in CH3CN at 45 °C | [474] |
| 9 | O | t-Bu |
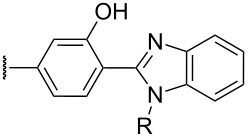 R = H, Me, Ph |
TBAF in CH3CN or NaOH in H2O at 45 °C | [475] |
| 10 | 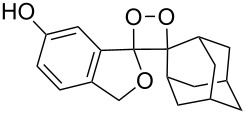 |
TBAF in DMSO at 25 °C | [470] | ||
| 11 | 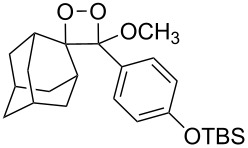 |
TBAF in THF/DMSO (1:1) at 25 °C | [476] | ||
| 12 | 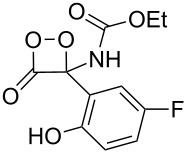 |
DBU in CH3CN at 25 °C | [477] | ||
| 13 | 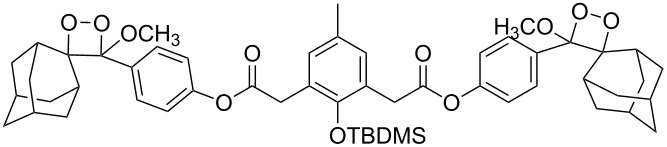 |
TBAF in DMSO/PBS buffer | [471] | ||
