Table 7.
Asymmetric oxidation of 3-substituted cyclobutanones 96a–f.
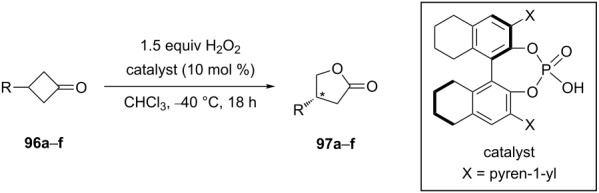 | |||
| Ketone | R | Yield, % | ee,% (conf.) |
| 96a | C6H5 | 99 | 88 (R) |
| 96b | 4-MeC6H4 | 99 | 93 (R) |
| 96c | 4-FC6H4 | 99 | 84 (R) |
| 96d | 2-naphthyl | 91 | 86 (R) |
| 96e | C6H5CH2 | 99 | 58 (S) |
| 96f | 4-MeOC6H4CH2 | 99 | 57 (S) |
Asymmetric oxidation of 3-substituted cyclobutanones 96a–f.
 | |||
| Ketone | R | Yield, % | ee,% (conf.) |
| 96a | C6H5 | 99 | 88 (R) |
| 96b | 4-MeC6H4 | 99 | 93 (R) |
| 96c | 4-FC6H4 | 99 | 84 (R) |
| 96d | 2-naphthyl | 91 | 86 (R) |
| 96e | C6H5CH2 | 99 | 58 (S) |
| 96f | 4-MeOC6H4CH2 | 99 | 57 (S) |