Fig. 3.
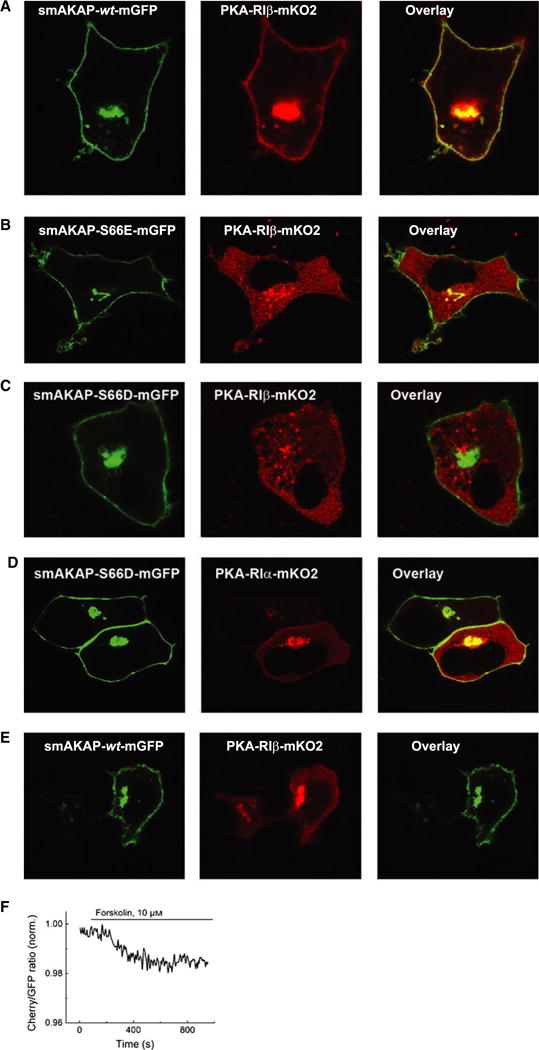
Phosphorylated smAKAP no longer localizes PKA-mRI at the plasma membrane. (A) Transfection of HeLa cells with smAKAP-wt-GFP and PKA-RIβ-mKO2 show colocalization at the plasma membrane. (B) In contrast, in the HeLa cells transfected with the phosphomimetic smAKAP-S66E-mGFP and PKA-RIβ-mKO2, no colocalization occurs. (C) After adding 8-CPT-cAMP and calyculin A, smAKAP-wt-mGFP is partially phosphorylated upon Ser66 by PKA-C, thus releasing PKA-RIβ-mKO2 into the cytosol. (D) A FRET-based kinetic assay was employed upon eight cells transfected with smAKAP-S66-mGFP, PKA-RIβ-mKO2, and PKA-C-HA. Upon stimulation with forskolin, there is a − 2.3 ± 0.4% reduction in FRET.
