Abstract
Nitrate-nitrogen is a common contaminant of drinking water in many agricultural areas of the United States of America (USA). Ingested nitrate from contaminated drinking water has been linked to an increased risk of several cancers, specific birth defects, and other diseases. In this research, we assessed the relationship between animal feeding operations (AFOs) and groundwater nitrate in private wells in Iowa. We characterized AFOs by swine and total animal units and type (open, confined, or mixed), and we evaluated the number and spatial intensities of AFOs in proximity to private wells. The types of AFO indicate the extent to which a facility is enclosed by a roof. Using linear regression models, we found significant positive associations between the total number of AFOs within 2 km of a well (p trend < 0.001), number of open AFOs within 5 km of a well (p trend < 0.001), and number of mixed AFOs within 30 km of a well (p trend < 0.001) and the log nitrate concentration. Additionally, we found significant increases in log nitrate in the top quartiles for AFO spatial intensity, open AFO spatial intensity, and mixed AFO spatial intensity compared to the bottom quartile (0.171 log(mg/L), 0.319 log(mg/L), and 0.541 log(mg/L), respectively; all p < 0.001). We also explored the spatial distribution of nitrate-nitrogen in drinking wells and found significant spatial clustering of high-nitrate wells (> 5 mg/L) compared with low-nitrate (≤ 5 mg/L) wells (p = 0.001). A generalized additive model for high-nitrate status identified statistically significant areas of risk for high levels of nitrate. Adjustment for some AFO predictor variables explained a portion of the elevated nitrate risk. These results support a relationship between animal feeding operations and groundwater nitrate concentrations and differences in nitrate loss from confined AFOs vs. open or mixed types.
Keywords: Nitrate, drinking water, private wells, animal feeding operations, generalized additive models, spatial cluster, spatial clustering
Graphical abstract
1. Introduction
Nitrate-nitrogen, referred to as nitrate throughout this paper, is one of the most common anthropogenic contaminants in groundwater and has been found in concentrations greater than the United States Environmental Protection Agency (EPA) maximum contaminant level (MCL) (10 mg/L as N) in 4.4% of private wells in the United States of America (USA) (DeSimone et al., 2009) and in 22% of private wells in agricultural areas (Dubrovsky et al., 2010). The MCL was promulgated in 1976 to protect infants from methemoglobinemia, an acute health effect. However, nitrate is a precursor in the endogenous formation of N-nitroso compounds (NOC), many of which are potent animal carcinogens and teratogens (IARC, 2010). Over the past 30 years, epidemiological studies have evaluated drinking water nitrate ingestion and risk of specific cancers, adverse reproductive outcomes, thyroid disease, and other chronic health effects (Ward et al., 2005; Ward, et al., 2010; IARC, 2010; Aschebrook-Kilfoy et al., 2012). Although the results of these studies are not conclusive, increased risk of colon cancer (De Roos et al., 2003), kidney cancer (Ward et al., 2007), ovarian cancer (Inoue-Choi et al., 2014), and neural tube defects (Brender et al., 2004; Brender et al., 2013) were found at nitrate concentrations less than the MCL especially among the population with dietary patterns that result in increased endogenous formation of NOC (colon, kidney cancers) or use of drugs that can form NOC (birth defects).
Sources of nitrate in groundwater in agricultural regions include inorganic fertilizers, animal manure applied to the land surface, and atmospheric deposition of nitrogen oxide compounds (Puckett, 1995). One source of manure, animal feeding operations (AFOs), has increased in the United States over the last several decades as livestock and poultry production have moved to fewer and more concentrated facilities. This has resulted in regional differences in the distribution of farm animals across the United States. Iowa has the largest percentage of swine animal units in the USA (27% with an estimated 32 million tons of swine manure in 2007) whereas Texas has the highest percentage of beef cattle animal units (16% with an estimated 60 million tons of manure in 2007) (EPA, 2013). An animal unit is defined as an animal of a thousand pounds of live weight (USDA, 2015), or approximately one beef cow. A thousand head of beef cattle is synonymous with 700 dairy cows, 2,500 swine weighing more than 55 pounds, 125,000 broiler chickens, or 82,000 laying hens (USDA 2015). AFOs concentrate animals, feed, and waste products in a small area and increase the risk of contamination of water resources from excessive nutrients, e.g. nitrogen, phosphorus, and/or ammonia; microbial pathogens; and pharmaceuticals (Burkholder et al., 2007). The EPA refers to AFOs as concentrated animal feeding operations (CAFOs) once they house more than 1,000 animal units or have more than 300 animal units and meet certain conditions set by the individual states (USEPA, 2004). CAFOs are further classified by their size based on the number and types of animals they house. The Iowa Department of Natural Resources (DNR) classifies AFO facilities as open, confined, or mixed, which indicates the extent to which they are enclosed by roofs. Open AFOs either have no roof or are partially roofed with no vegetation in the animals’ confinement area, and mixed AFOs are a combination of totally confined areas and partially or no roofed areas (DNR, 2015). A nutrient management plan is required for confined AFOs with more than 500 animal unit capacity and for open AFOs with a National Pollutant Discharge Elimination System (NPDES) permit or a capacity of 1,000 animal units (DNR, 2015).
Prior studies have found elevated nitrate concentrations in wells less than 10 km from AFOs (Exner and Spalding, 1994; Michalopoulis et al., 2014; Toetz, 2006; and Lockhart et al., 2013). Monitoring wells 2 – 8 km downgradient of a cattle AFO in Nebraska had an average nitrate concentration of 11.3 mg/L, and N isotope analyses indicated both fertilizer and animal sources (Exner and Spalding, 1994). Land application of treated wastewater from a hog AFO in Crete contributed to nitrate-nitrogen concentrations up to 29 mg/L in monitoring wells downgradient 0.3 – 1.5 km (Michalopoulos et al., 2014). Nitrate concentrations in the winter were significantly greater than in the spring due to increased precipitation and leaching of nitrate to groundwater. Monitoring wells 0.5 – 1.5 km downgradient of a swine AFO in Oklahoma had elevated nitrate concentrations (10.9 – 26.1 mg/L) compared with upgradient wells (< 6.7 mg/L) (Toetz, 2006). Nitrate concentration and δ15NO3 were elevated in wells downgradient from the AFO, indicating that animal manure was the source of contamination (Toetz, 2006). In the Stanislaus-Merced area of the Central Valley, California, nitrate-nitrogen concentrations in private wells increased significantly with decreasing distance to a dairy corral or waste lagoon (Lockhart et al., 2013). However, proximity to a dairy corral was not always associated with high nitrate in the study area due to complicating factors such as groundwater flow direction, hydraulic gradient, depth to groundwater, and historical land use.
The swine industry in Iowa has undergone major changes since about 1980 (Honeyman and Duffy, 2006; Melvin et al., 2002). Between 1978 and 2002, the average number of pigs on a farm increased by 600% from 250 pigs to more than 1,500 pigs. Simultaneously in that timeframe, the number of pig farms in Iowa decreased by 83% from 59,000 pig farms to 10,205 (Honeyman and Duffy, 2006). Although the total number of swine in Iowa was fairly constant during the 1980s and 1990s, the number of farms with swine declined resulting in an increase in the average number of swine per farm (Melvin et al., 2002). This resulted in an increased concentration of waste in fewer, larger feeding operations. The spatial distribution of the swine population also changed over time in Iowa. Prior to 1980, most Iowa counties had substantial numbers of hogs, but as of 2002, hogs were mostly concentrated in northwestern and selected southeastern counties (Honeyman and Duffy, 2006).
The increased concentration of AFO waste in Iowa has resulted in an increased potential for nitrate contamination of private wells. Manure N has been significantly and positively correlated with groundwater nitrate concentrations in previous modeling studies. In a study in the USA, manure from CAFOs was one of several N sources evaluated in nonlinear regression models and was highly significant (Nolan and Hitt, 2006). In a study in Spain, manure from cattle and hogs was the only significant N source that predicted ground water nitrate levels in a regression model (p = 0.0013) (Boy-Roura et al., 2013). Incorporation of AFO variables in such models is rare. One recent study in North Carolina found that swine CAFOs and swine lagoons were significant predictors of nitrate levels in monitoring wells and private wells, respectively (p < 0.025 for both) (Messier et al., 2014). In Iowa, Wheeler et al. (2015) found the distance to the nearest AFO and the number of animal units at the nearest AFO to be important predictors of log nitrate in a random forest model. In the present study, we assessed the relationship between AFOs and nitrate concentrations in private wells. We characterized AFOs by animal type (all animals and swine only) and whether they were open, confined, or mixed. In addition, we explored the spatial distribution of high-nitrate (>5 mg/L) well measurements relative to low-nitrate well measurements (≤ 5 mg/L) by testing for spatial clustering and modeling the spatial variation in risk of a high-nitrate well using generalized additive models, both before and after adjusting for AFO variables. To the best of our knowledge, this is the first study to assess the relationship between groundwater nitrate and several measures of AFOs including measures stratified by AFO type. . The results of this study may be applicable in areas with similar environmental issues outside the USA.
2. Materials and methods
2.1 Private well nitrate measurements and characteristics
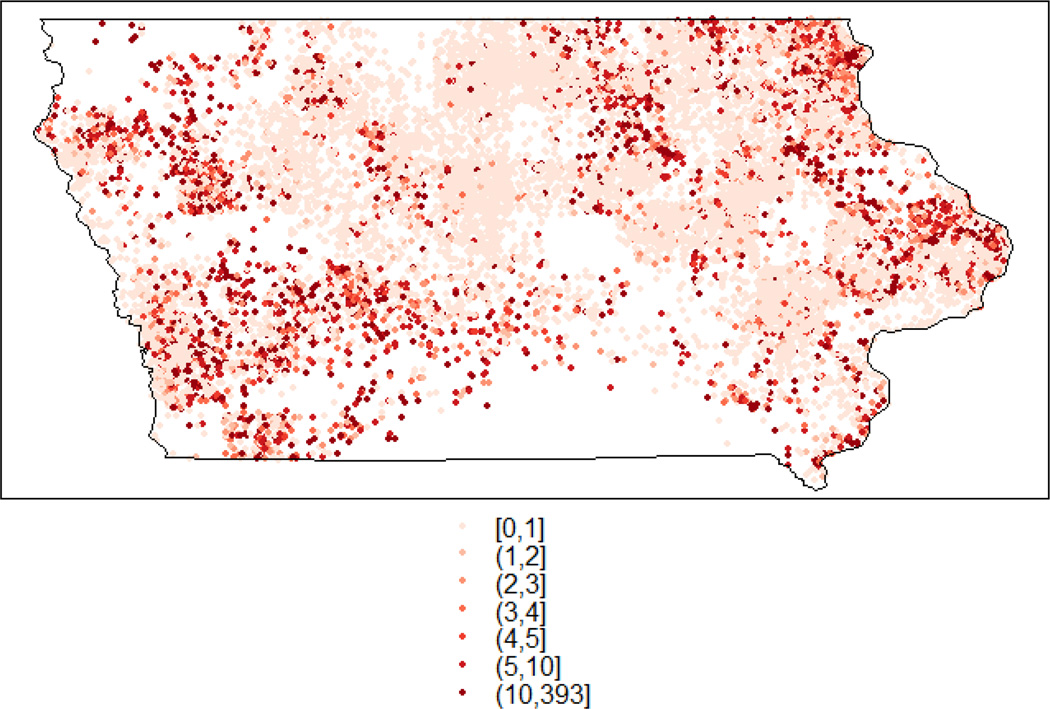
As previously described (Wheeler et al., 2015), we obtained 34,084 nitrate measurements from private drinking water wells sampled between 1980 and 2011 from all counties in Iowa. The wells were completed either in unconsolidated sediments or bedrock depending on the quality of the aquifer and water availability. Nitrate measurements were reported either as nitrate-nitrogen or nitrite-plus-nitrate nitrogen, and the latter was converted to nitrate-nitrogen. We restricted our analysis to well measurements between 2001 and 2011 (n=27,455) to more closely align with the time period of our AFO data. Of the measurements, 2,162 were above the EPA MCL of 10 mg/L (7.9%). A map of nitrate measurements and locations is displayed in Figure 1.
Figure 1.
Nitrate measurements (mg/L nitrate-nitrogen) for (n=27,455) private well locations in Iowa, USA.
We used a geographic information system (GIS) to create AFO measures and to characterize properties of the wells. We obtained data for the 9,057 AFOs operating between 2006 and 2011 that were registered, permitted, or monitored by the Iowa Department of Natural Resources. The dataset is not a complete census of AFOs in Iowa, but the percentage of animals in AFOs that are not included is small (Gene Tinker, personal communication, December 17, 2015). The dataset included information on operation type, specific animal units, and geographic location. For operation type, there were 366 mixed (4%), 6,778 confined (75%), and 1,913 open (21%) AFOs. We computed six AFO variables at varying, circular buffer distances from the private well locations: total animal units, swine units, number of total AFOs within a buffer, and number of open, confined, and mixed AFOs within a buffer. We focused specifically on swine units based on their predominance at AFOs, especially confined AFOs (Table 1). Swine accounted for 86% of all animal units in confined AFOs in Iowa and 73% of all animal units considering all three types of AFOs (Table 1). Johnson and Belitz suggest “circular area surrogates” are appropriate buffers for predictor variables when a physically based approach such as a numerical flow model to determine the contributing land surface area to a well is not feasible (2009). Buffer distances were chosen based on previous values used in the groundwater literature and included 500 m, 1 km, 2 km, 10 km, 15 km, 20 km, 30 km, and 50 km (Johnson and Belitz, 2009; Wheeler et al., 2015).
Table 1.
Animal units by type of AFO for AFOs operating between 2006 and 2011 that were registered, permitted, or monitored by the Iowa Department of Natural Resources. Percentages correspond to total animal units within each AFO type.
| Animal Type | Units for All AFOs |
Units for Open AFOs |
Units for Confined AFOs |
Units for Mixed AFOs |
|---|---|---|---|---|
| Swine | 7,389,541 (73%) | 33,754 ( 3%) | 7,186,783 (86%) | 169,004 (30%) |
| Dairy Cattle | 226,195 ( 2%) | 34,453 ( 3%) | 104,054 ( 1%) | 86,688 (15%) |
| Beef Cattle | 1,466,881 (15%) | 1,083,229 (94%) | 70,417 ( 1%) | 313,235 (55%) |
| Chickens | 945,345 ( 3%) | 13 ( 0%) | 945,228 (11%) | 104 ( 0%) |
| Turkeys | 75,762 ( 1%) | 0 ( 0%) | 73,506 ( 1%) | 2,256 ( 0%) |
| Horses | 6,678 ( 0%) | 2,832 ( 0%) | 3,624 ( 0%) | 222 ( 0%) |
| Sheeps, Lambs, and Goats | 3,661 ( 0%) | 2,098 ( 0%) | 315 ( 0%) | 1,358 ( 0%) |
| Totals | 10,109,063 | 1,156,379 | 8,383,927 | 572,867 |
To consider properties of the wells and the well environment, we considered a set of 65 explanatory variables, where many were calculated using a GIS and circular buffers of various sizes (see Wheeler et al. 2015 for more details). Briefly, the variables included proximity to karst terrain, soil type and other soil variables, climate, geology, groundwater vulnerability, and aquifer characteristics. Certain variables represented measurements going as far back as 1978, including proportion of agricultural land within a 1 km buffer and annual county-level N fertilizer within 1 km. The variables represented the major N sources to the land surface, factors affecting the transport of nitrogen through soils and rocks to wells, and variables relating to denitrification (e.g. soil drainage as represented by soil variables such as organic matter and depth to seasonally high water table) (Nolan and Hitt, 2006). From this set of variables, we determined the variables for which to adjust in linear regression models for nitrate that included AFO measures.
2.2 Statistical Analysis
To select the well environment variables to include in regression models to evaluate AFO measures and log nitrate, we used Least Absolute Shrinkage and Selection Operator (LASSO) and forward stepwise regression models. Forward stepwise regression chooses variables that improve model fit and LASSO shrinks coefficients of regression terms toward zero in cases of strong correlation with other covariates. Variables were included in the subsequent models if they were selected by both LASSO and forward stepwise regression. Boy-Roura et al. (2013) previously used similar stepwise methods for variable selection. To avoid removing a large proportion of the observations, we excluded 25 of the 65 candidate adjustment variables that had more than 50% missing values. Missing values in the remaining variables was either imputed as 0 if the variable values included 0 or the variable was categorized and included a category for missing data. In total, we identified 15 variables as important covariates in models for log nitrate (Table 2). These covariates include karst category, groundwater vulnerability region category, and well depth and can be considered surrogates for aquifer variables. The vulnerability region category represents the degree of drift confinement of bedrock aquifers, proximity of sinkholes, presence of agricultural drainage wells, and locations of alluvial aquifers. Well depth can also be considered a proxy for groundwater age and redox condition. The likelihood of being in a confined bedrock aquifer with older, more reduced groundwater correspondingly increases with well depth. Furthermore, there were only two cases where aquifer variables were significant (silt and water depth), and when linear regression were fit with and without the aquifer variables, there were insignificant differences in the estimates and significance of the AFO variables.
Table 2.
Well variables determined to be important covariates for log nitrate concentrations in Iowa private wells. More details on the sources for the variables are available in Wheeler et al. (2015).
| Variable(s) | Definition |
|---|---|
| Well Depth | Depth of the nitrate sampling well (0.305 m or ft). |
| Nitrogen Fertilizer use, 2000 and 2002 | Annual 2000 and 2002 county-level nitrogen fertilizer (kg of N/year) apportioned to 2000 and 2002 agricultural lands within a 1 km well buffer. |
| Agricultural Land 1978 and 2002 | Agricultural land in 1978 and 2002 within a 1 km radius well buffer, expressed as a percentage. |
| Slope | Average percent slope within a 1 km buffer. |
| Minimum Temperature | Mean annual minimum temperature at well point for the time period 1981–2010 (degrees Celsius times 100). |
| Karst Category | Karst categorical value at well point. Indicates areas within one mile of known sinkholes as well as areas that have carbonate bedrock within 50 feet of the land surface. Categories included no karst, less than a 304.8 m (1000 ft.) sink, and greater than a 304.8 m (1000 ft.) sink. |
| Groundwater Region Category | Groundwater vulnerability region at well point broken into categories that characterized wells by bedrock, shale, and drainage composition. |
| USGS Well Logs | Categorical variable created from grouping the number of wells reported to the USGS, including location, ownership, and construction details, within a 4×4 mile square around the well point. Grouping is based on general known accuracy of variables created from contributing well log data (e.g., higher well log counts indicate increased accuracy). |
| Fine-Grain Deposit Thickness | Total thickness of fine-grain soil deposits at the well (0.305 m or ft). Values were calculated from above the top of the measurement well screen (assumed to be the well depth minus three feet) using an interpolated grid created from well log data within a 6.44×6.44-km (4×4-mile) square around the well point. |
| Well Logs at 9.66 km | Number of USGS well logs within a 9.66×9.66-km (6×6-mile) square around the well point. |
| Well Logs at 6.44 km | Number of USGS well logs within a 6.44×6.44-km (4×4-mile) square around the well point. |
| Horizontal Hydraulic Conductivity | Average horizontal hydraulic conductivity of all glacial deposits within a 6.44×6.44-km (4×4-mile) square around the well point (0.304 m or ft/day). |
| Saturated Hydraulic Conductivity | Average soil saturated hydraulic conductivity within a 1 km radius well buffer (micrometers/sec) |
We determined the optimal buffer distance for each AFO variable by modeling log nitrate adjusting for each of the variables in Table 2 in individual linear regression models for each buffer size candidate. For each AFO variable, we chose the model with the lowest Akaike’s Information Criterion (AIC) (Akaike, 1973) to select the buffer size. AIC allows model comparison by estimating the relative loss of information between models. If the AIC continually decreased as the buffer size increased, then we selected the buffer distance when the AFO variable first became significant in the models for log nitrate.
We evaluated the assumption of linearity between log nitrate and each AFO-related variable using generalized additive models (GAMs) and a thin plate regression spline (TPRS) smoothing function for each AFO-related variable, which allows for nonlinear relationships between the dependent and independent variables (Wood, 2006). After finding the relationship between the AFO variables and log nitrate to be nonlinear, we computed quartiles for each variable. The variables that had more than a quarter of their values equal to zero had a reference level created that included all observations with zeroes. The remaining observations were calculated as tertiles. These variables included swine units, total AFOs, and open AFOs. We used the quantiles in the final linear regression models with one model for each AFO variable and calculated tests of trend for the AFO variables’ quantiles.
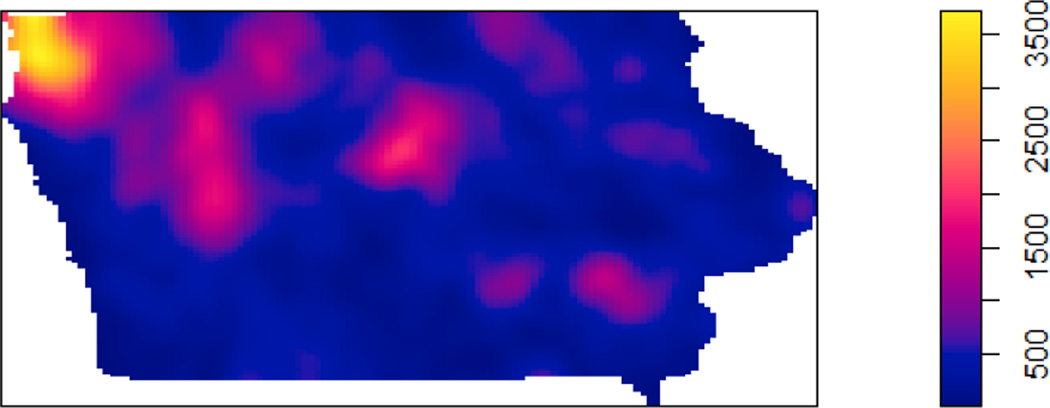
In addition to using the counts of AFOs within buffer distances, we considered the AFO locations as a spatial point pattern and computed spatial intensities of all AFOs as well as by AFO type. The spatial intensity estimates the mean number of AFOs at a particular point in space using a Gaussian kernel function with bandwidths selected to minimize the mean squared error (Silverman, 1986). A map of all AFO intensity shows that the intensity of AFOs was highest in the northwest corner of the state and was also high in a few areas of central Iowa (Figure 2). Similar patterns were observed with open and mixed AFO intensity maps (not shown). We evaluated the linearity assumption for the AFO intensities as described above and found them to be nonlinear with log nitrate. Hence, we entered the intensities as quartiles in the final linear regression models.
Figure 2.
Spatial distribution of AFO intensity in Iowa.
To investigate the spatial distribution of wells with higher versus lower nitrate concentrations, we considered the wells as a marked spatial point pattern with well measurements designated as high-nitrate where nitrate-nitrogen was > 5 mg/L and as low-nitrate otherwise. With this classification, our dataset contained 4,414 high-nitrate well measurements (16%) and 23,041 low-nitrate well measurements (84%). We evaluated whether high-nitrate measurements tended to cluster spatially when compared with low-nitrate measurements at distances up to 30 km using the difference in the K-functions for high-nitrate and low-nitrate wells (Diggle and Chetwynd, 1991). We assessed the significance of spatial clustering at particular distances and overall using Monte Carlo randomization with 999 iterations (Baddeley et al., 2014).
To model the spatial variation in risk of being a high-nitrate well, we used binary GAMs with a bivariate TPRS over the spatial coordinates for wells (Kelsall and Diggle, 1998; Wood, 2006). These models estimated the spatial log-odds of being a high-nitrate well and identified areas with statistically significant increased odds of being a high-nitrate well in Iowa. GAMs were similarly used by Mellor and Cey (2015) to look at nitrate vulnerability in agricultural aquifers. We estimated a crude model and a model adjusted for well depth, total animal units within 10 km of a well, total open AFOs within 5 km of a well, and total AFOs within 2 km of a well. The AFO variables were included in the adjusted model to determine if they would contribute to explaining the spatial log-odds of being a high-nitrate well. Well depth was also included given its importance in predicting nitrate (Wheeler et al., 2015). Significance of the spatial log-odds was evaluated with Monte Carlo randomization and 999 simulations over a 500x500 grid covering Iowa. Areas of significantly increased log-odds of having high nitrate (points that rank in the upper 2.5% of the pointwise permutation distribution of the spatial log-odds) were identified on a 500x500 map of the spatial log-odds of being a high-nitrate well. We conducted all analyses in the R computing environment (R Development Core Team, 2006). We used the library mgcv (Wood, 2006) for modeling the log-odds of being a high-nitrate well and the library spatstat (Baddeley and Turner, 2005) for testing for spatial clustering.
3. Results and Discussion
The optimal buffer distance varied for the six AFO count variables. The optimal buffer distances were 10 km for total animal units, 2 km for swine units and total AFO counts, 5 km for open AFO counts, 6 km for confined AFO counts, and 30 km for mixed AFO counts. For swine units, a lower AIC was found with the largest larger buffer distance (30 km) compared to smaller distances, but the effect estimates were not consistent when a GAM was fitted to model residual spatial autocorrelation. Swine unit effect estimates from a linear regression model and a GAM using a buffer distance of 2 km were consistent. Messier et al. also found a positive relationship between nitrate and counts of swine CAFOs within this buffer distance (2014). For mixed AFO counts, statistical significance was found starting at 500 m, but the AIC was lowest at 30 km. This may reflect the widespread reach of nitrate runoff.
We show the results of linear regression models for quantiles of the AFO variables in Table 3. We found a significant positive trend between the log nitrate concentration and total AFO counts within 2 km (p < 0.001), open AFO counts within 5 km (p < 0.001), and mixed AFO counts within 30 km (p < 0.001). Compared to having no open AFOs within 5 km, the highest quantile of open AFOs within 5 km was associated with an increase of 0.622 log(mg/L) in nitrate (p < 0.001). Compared to the first quartile, the fourth quartile of mixed AFOs within 30 km was associated with a 0.232 log(mg/L) increase (p < 0.001). Relative to having no AFOs within 2 km, the third and fourth quartiles of total AFO counts were significantly associated with 0.144 and 0.131 log(mg/L) increases (p < 0.001 and p = 0.003), respectively. Comparing the individual model AICs, the model with open AFOs within 5 km had the lowest AIC followed by the model with mixed AFOs within 30 km. There was little evidence of an association with confined AFOs within 6 km (p trend = 0.187) in spite of the fact that confined AFOs composed the majority of AFOs in Iowa. This result may be related to the fact that confined AFOs are required to retain all their manure and dispose of it via land application and other methods. Confined AFOs also house the majority of swine (Table 1), which output less nitrogen compared to other animals such as cattle (Goolsby et al., 1999).
Table 3.
Results from linear regression models for the relationship between AFO measures and nitrate-nitrogen log(mg/L) in private wells in Iowa.
| Variable | Quantiles | Coefficient | Standard Error |
P-Value | Trend Test | |
|---|---|---|---|---|---|---|
| Animal Units | Q2: | [4471, 10651] | 0.071 | 0.030 | 0.015* | |
| within 10 km | Q3: | [10652, 22988] | 0.075 | 0.030 | 0.013* | |
| Q4: | [22989, 199218] | 0.002 | 0.032 | 0.952 | 0.144 | |
| Swine Units | Q2: | [4,992] | 0.153 | 0.037 | < 0.001* | |
| within 2 km | Q3: | [993, 1992] | 0.034 | 0.038 | 0.374 | |
| Q4: | [1993, 18600] | −0.053 | 0.038 | 0.164 | 0.943 | |
| Total AFOs | Q2: | [1] | 0.093 | 0.028 | < 0.001* | |
| within 2 km | Q3: | [2] | 0.144 | 0.041 | < 0.001* | |
| Q4: | [3, 12] | 0.131 | 0.045 | 0.003* | < 0.001* | |
| Open AFOs | Q2: | [1] | 0.081 | 0.027 | 0.002* | |
| within 5 km | Q3: | [2] | 0.222 | 0.037 | < 0.001* | |
| Q4: | [3, 21] | 0.622 | 0.040 | < 0.001* | < 0.001* | |
| Confined AFOs | Q2: | [2, 3] | 0.070 | 0.029 | 0.015* | |
| within 6 km | Q3: | [4, 6] | 0.038 | 0.031 | 0.215 | |
| Q4: | [7, 44] | 0.042 | 0.029 | 0.154 | 0.187 | |
| Mixed AFOs | Q2: | [2] | 0.063 | 0.030 | 0.036* | |
| within 30 km | Q3: | [3,4] | 0.033 | 0.029 | 0.250 | |
| Q4: | [5, 126] | 0.232 | 0.029 | < 0.001* | < 0.001* | |
| Intensity for Total | Q2: | [0.10, 0.70] | 0.030 | 0.031 | 0.330 | |
| AFOs | Q3: | [0.71, 734.00] | 0.109 | 0.032 | < 0.001* | |
| Q4: | [734.00, 7847.00] | 0.171 | 0.034 | < 0.001* | < 0.001* | |
| Intensity for Open | Q2: | [0.0, 22.0] | −0.013 | 0.030 | 0.678 | |
| AFOs | Q3: | [22.1, 109.0] | 0.056 | 0.031 | 0.070 | |
| Q4: | [109.1, 2552.0] | 0.319 | 0.032 | < 0.001* | < 0.001* | |
| Intensity for | Q2: | [1.2, 25.0] | 0.050 | 0.031 | 0.110 | |
| Confined AFOs | Q3: | [25.1, 492.0] | 0.047 | 0.033 | 0.145 | |
| Q4: | [492.1, 5975.0] | 0.050 | 0.035 | 0.156 | 0.205 | |
| Intensity for | Q2: | [0.000, 0.001] | 0.304 | 0.031 | < 0.001* | |
| Mixed AFOs | Q3: | [0.002, 2.590] | 0.333 | 0.031 | < 0.001* | |
| Q4: | [2.600, 1166.880] | 0.541 | 0.032 | < 0.001* | < 0.001* | |
denotes p-value < 0.05
The number of animal units within 10 km was positively associated with log nitrate in the second, third, and fourth quartiles, with the increases of 0.071 and 0.075 log(mg/L) in nitrate for the second and third quartiles being statistically significant (p=0.015 and p=0.013, respectively). Conversely, we found little evidence of an association between log nitrate and quartiles of swine units within 2 km (p = 0.43). However, in a model with a binary indicator for whether or not there were swine units within 2 km, we found a significant positive association (0.049 log(mg/L), p = 0.045). In general, the larger association with animal units maybe attributed to cattle manure having a higher nitrogen content than that of hogs and pigs according to nutrient budgets of the region (Goolsby et al., 1999; Ruddy et al., 2006). We may also have found a larger association with swine units if we were able to evaluate the locations of liquid injection of swine manure into soils. This practice has become more common with the increase in AFOs and can substantially increase nitrate loss from the soils compared with use of urea ammonium nitrate fertilizer (Baksh et al., 2005).
The results from evaluating AFO spatial intensities were similar to the results with the AFO counts. Specifically, the intensities for all, open, and mixed AFOs had significant trends with log nitrate (p < 0.001 for all three). There was no evidence of an association between log nitrate and intensity for confined AFOs (p = 0.205). For all intensities except the second quartile for open AFOs, the relationship was positive. We observed statistically significant increases in the third (0.109 log(mg/L)) and fourth (0.171 log(mg/L)) quartiles of total AFO intensity, and open AFO intensity was associated with a significant 0.319 log(mg/L) increase in log nitrate in the fourth quartile (p < 0.001). Mixed AFO intensity was also significantly associated with increases in log nitrate (p < 0.001 for all quartiles). Mixed AFOs in Iowa are classified as operations with some animals in confinement and others in open feedlots. The lack of observed relationship for confined AFOs, but similar relationships for mixed and open AFOs intensities may reflect the greater likelihood of groundwater contamination even without explicit buffer distances. The contamination is most likely a function of runoff and leaching or inadvertent waste discharge to sinkholes or agricultural drainage wells in the operations. In light of similar results from AFO counts, these data generally support a relationship between non-confined animal feeding operations and groundwater nitrate concentrations. To our knowledge, this is the first study to evaluate the association between AFO types and nitrate. Even though we found little evidence of an association with confined AFOs, we still expect there to be nitrate output from these operations. Our models did not address the land application of manure offsite, which could be a significant source of nitrate to groundwater, as Nolan and Hitt find a significant positive relationship between manure and groundwater nitrate (2006). Well measurements were also treated as conditionally independent after adjusting for the covariates in Table 2.
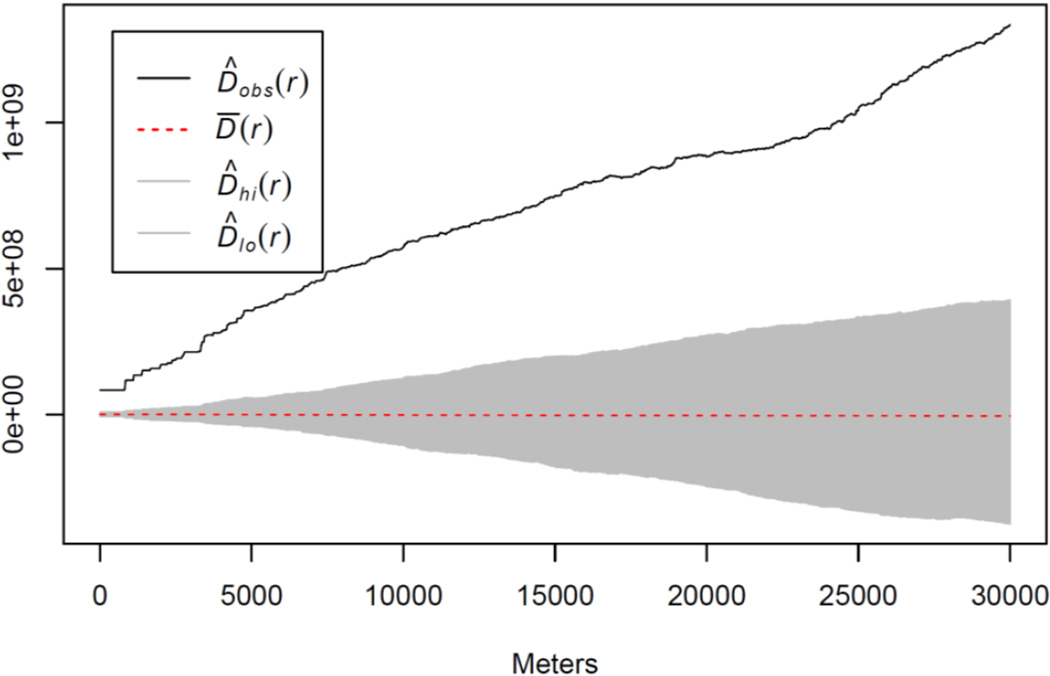
Considering the spatial distribution of high-nitrate and low-nitrate wells, we found evidence of significant spatial clustering in high-nitrate wells at distances up to 30 km (Figure 3). The difference in the K-functions between high-nitrate and low-nitrate wells was consistently and significantly greater than expected under the null hypothesis of equal distribution for high-nitrate and low-nitrate wells up to this distance (p = 0.001). The significant spatial clustering of high-nitrate wells suggests that there are common hydrogeological and/or agricultural land-use characteristics leading to local clustering of wells with high nitrate.
Figure 3.
Results from testing for spatial clustering of high-nitrate wells relative to low-nitrate wells. The y-axis is the difference in K-functions between high- and low-nitrate wells at particular distances specified on the x-axis. The gray area is a pointwise envelope that reflects the variability in the difference in K-functions assuming high-nitrate and low-nitrate wells have the same spatial distribution. The envelope is centered about D̄(r), the theoretical mean difference in the K-functions for high- and low-nitrate wells at a particular distance r, and bounded by D̂lo(r) and D̂hi(r), the critical points from a Monte Carlo test at significance level 0.05. D̂obs(r) is the difference in the K-functions between high-nitrate and low-nitrate wells for the observed data.
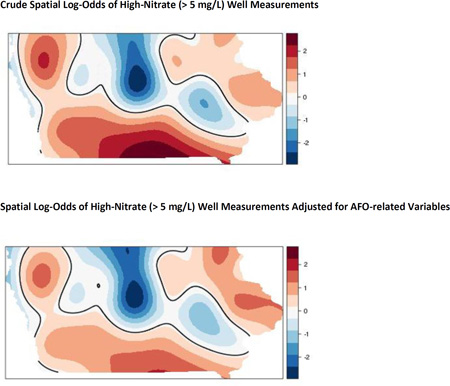
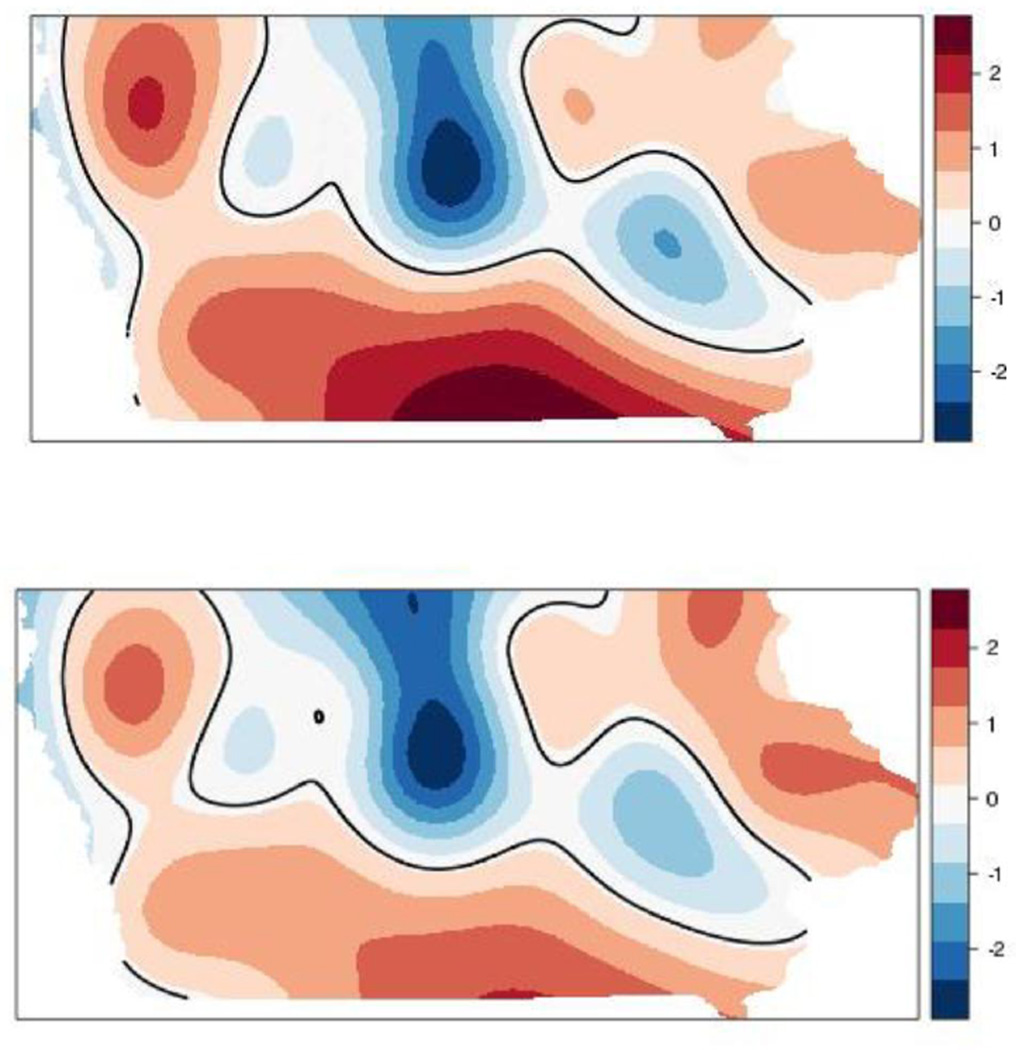
We also found statistically significant areas of high-nitrate wells with the GAMs. There were significantly higher spatial log-odds of being a high-nitrate well in the south and in the northwest corner of Iowa based on the crude GAM (top panel of Figure 4). The northwest corner aligns with the pattern of AFO intensity (Figure 2). Conversely, central Iowa had decreased log-odds of having nitrate > 5 mg/L, which may be affected by what is known as the Des Moines Lobe, where receding glaciers deposited low permeability sediments (Miller et al., 2009). Adjusting for well depth, total animal units within 10 km, total AFO count within 2 km, and open AFOs within 5 km, the log-odds of being a high-nitrate well (bottom panel of Figure 4) decreased in the south and northwest corner of the state relative to the crude model, and increased slightly in the northeast part of the state, which coincides with karst geology. There continued to be decreased log-odds of high-nitrate wells in central Iowa after adjusting for the covariates. Overall, the AFO-related variables explained a substantial amount of the spatial log-odds of being a high-nitrate well when comparing the statistically significant areas in Figure 4. Other factors contributing to nitrate aside from AFOs (i.e., soil, geologic, and farming practices) remain to be explored to explain the higher risk area for high-nitrate wells in the eastern part of the state. Future analyses should investigate if hydrogeological characteristics affect the relationship between nitrate and AFO. Different methodology may be necessary to handle collinearity issues that are expected when controlling for numerous suspected confounders.
Figure 4.
Crude spatial log-odds of high-nitrate (> 5 mg/L) well status (top panel) and adjusted spatial log-odds accounting for well depth, total animal units within 10 km, total AFOs within 2 km, and open AFOs within 5 km (bottom panel). The black lines indicate statistically significant areas of risk of being a high-nitrate well.
Overall, the buffer distance results are consistent with previous work by Wheeler et al. (2015) and other literature. All buffer distances for AFOs except for one (mixed AFOs) are less than 10 km, which agrees with the previous findings of Exner and Spalding (1994), Michalopoulis et al. (2014) Toetz (2006), and Lockhart et al. (2013). Animal units were also found to be significantly associated with log nitrate similar to Boy-Roura et al. (2013).
4. Conclusion
In this research, we assessed the associations between measures of AFOs and nitrate concentrations in private drinking water wells in Iowa. To our knowledge, this is the first study to evaluate the association between nitrate and AFOs by type (i.e., open, confined, mixed). Open and mixed AFOs were significantly positively associated with nitrate concentrations. Confined AFOs explained less of the variation in log nitrate. Additionally, we evaluated spatial clustering of private wells by nitrate level and found that high nitrate wells (>5 mg/L nitrate-nitrogen) were significantly clustered compared with low nitrate wells. We also identified areas at greater risk of having high nitrate near Iowa’s southern border and northwest corner, which corresponded to the highest concentration of AFOs in northwest Iowa. Along with well depth, the number and type of AFOs explained a portion of the elevated risk of high-nitrate wells in these areas; however, significantly elevated risk remained unexplained. Considerations for future research include the evaluation of the mix of different animal types at a facility; land application of manure after on-site retention, especially the distance from wells to application sites; human contributions such as poorly maintained septic systems; and interactions of AFO variables with soil and geologic variables. In addition, more studies are needed in other geographic areas to further elucidate the relationship between the characteristics of AFOs and groundwater nitrate levels.
Highlights.
We assessed the relationship between animal feeding operations (AFOs) and groundwater nitrate in private wells in Iowa.
The total number of AFOs, open AFOs, and mixed AFOs were significantly and positively associated with log nitrate concentration in wells.
High nitrate-nitrogen wells (>5 mg/L) were significantly spatially clustered compared to low nitrate-nitrogen wells (≤ 5 mg/L).
Statistically significant areas of risk for high levels of nitrate in Iowa were partially explained by AFO predictor variables.
Acknowledgments
The project described was supported by Grant Number T32 ES007334 from the National Institute of Environmental Health Sciences (NIEHS). This study was also supported by the Intramural Research Program of the National Cancer Institute. The publication contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike H. Proceedings of the Second International Symposium on Information Thheory. Budapest, Hungary: Akademiai Kaido; 1973. Information theory and extension of the maximum likelihood priniciple; pp. 267–281. [Google Scholar]
- Aschebrook-Kilfoy B, Heltshe SL, Nuckols JR, Sabra MM, Schldiner AR, Mitchell BD, Airola M, Holford TR, Zhang Y, Ward MH. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the Old Order Amish in Pennsylvania. Environmental Health. 2012;11(6):1–11. doi: 10.1186/1476-069X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AJ, Turner R. Spatstat: An R package for analyzing spatial point patterns. Journal of Statistical Software. 2005;12(6):1–42. [Google Scholar]
- Baddeley A, Diggle PJ, Hardegen A, Lawrence T, Milne RK, Nair G. On tests of spatial pattern based on simulation envelopes. Ecological Monographs. 2014;84(3):477–489. [Google Scholar]
- Baksh A, Kanwar RS, Karlen DL. Effects of liquid swine manure applications on NO3-N leaching losses to subsurface drainage water from loamy soils in Iowa. Argriculture, Ecosystems and Environment. 2005;109(1–2):118–128. [Google Scholar]
- Boy-Roura M, Nolan BT, Menció A, Mas-Pla J. Regression model for aquifer vulnerability assessment of nitrate pollution in the Osona region (NE Spain) Journal of Hydrology. 2013;505:150–162. [Google Scholar]
- Brender JD, Olive JM, Felkner M, Suarez L, Marckwardt W, Hendricks KA. Dietary nitrites and nitrates, nitrosatable drugs, and neural tube defects. Epidemiology. 2004;15(3):330–336. doi: 10.1097/01.ede.0000121381.79831.7b. [DOI] [PubMed] [Google Scholar]
- Brender JD, Weyer PJ, Romitti PA, Binayak PM, Shinde MU, Vuong AM, Sharkey JP, Dwivedi D, Horel SA, Kantamneni J, Huber JC, Jr, Zheng Q, Werler MM, Kelley KE, Griesenbeck JS, Zhan FB, Langlois PH, Suarez L, Canfield MA the National Birth Defects Prevention Study. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the National Birth Defects Prevention Study. Environmental Health Perspectives. 2013;121(9):1083–1089. doi: 10.1289/ehp.1206249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder J, Libra B, Weyer P, Heathcote S, Kolpin D, Thorne PS, Wichman M. Impacts of waste from concentrated animal feeding operations on water quality. Environ Health Perspect. 2007;115(2):308–312. doi: 10.1289/ehp.8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos AJ, Ward MH, Lynch CF, Cantor KP. Nitrate in public water supplies and the risk of colon and rectum cancers. Epidemiology. 2003;14(6):640–649. doi: 10.1097/01.ede.0000091605.01334.d3. [DOI] [PubMed] [Google Scholar]
- DeSimone LA, Hamilton PA, Gilliom RJ. The quality of our nation’s waters—Quality of water from domestic wells in principal aquifers of the United States, 1991–2004—Overview of major findings. 2009 [Google Scholar]
- Diggle PJ, Chetwynnd AG. Second-Order Analysis of Spatial Clustering for Inhomogenous Populations. Biometrics. 1991;47(3):1155–1163. [PubMed] [Google Scholar]
- DNR. [accessed in November 2015];Animal Feeding Operations. 2015 available at http://www.iowadnr.gov/Environmental-Protection/Land-Quality/Animal-Feeding-Operations.
- Dubrovsky NM, Burow KP, Clark GM, Gronberg JM, Hamilton PA, Hitt KJ, Mueller DK, Munn MD, Nolan BT, Puckett LS, Rupert MG, Short TM, Spahr NE, Sprague LA, Wilber WG. The quality of our Nation’s waters—Nutrients in the Nation’s streams and groundwater, 1992–2004. U.S. Geological Survey; 2010. p. 1350. [Google Scholar]
- EPA. Literature Review of Contaminants in Livestock and Poultry Manure and Implications for Water Quality, EPA 820-R-13-002. 2013:125. [Google Scholar]
- Exner ME, Spalding RF. N-15 Identification of nonpoint sources of nitrate contamination beneath cropland in the Nebraska Panhandle: Two case studies. Applied Geochemistry. 1994;9(1):73–81. [Google Scholar]
- Goolsby DA, Battaglin WA, Lawrence GB, Artz RS, Aulenbach BT, Hooper RP, Keeney DR, Stensland GJ. Flux and sources of nutrients in the Mississippi-Atchafalaya River Basin—Topic 3 report for the integrated assessment on the hypoxia in the Gulf of Mexico. Silver Spring, Md., NOAA Coastal Ocean, NOAA Coastal Ocean Program Decision Analysis Series no. 17. 1999:1–151. [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Ingested nitrate and nitrite and cyanobacterial peptide toxins. World Health Organization, International Agency fr Research on Cancer. 2010;94 [PMC free article] [PubMed] [Google Scholar]
- Honeyman MS, Duffy MD. Iowa’s Changing Swine Industry. Animal Industry Report AS. 2006;652:ASL R2158. [Google Scholar]
- Johnson TD, Belitz K. Assigning land use to supply wells for the statistical characterization of regional groundwater quality: correlating urban land use and VOC occurrence. Journal of Hydrology. 2009;370(1–40):100–108. [Google Scholar]
- Kelsall JE, Diggle PJ. Spatial variation in risk of disease: a nonparametric binary regression approach. Applied Statistics. 1998;47:559–573. [Google Scholar]
- Lockhart KM, King AM, Harter T. Identifying sources of groundwater nitrate contamination in a large alluvial groundwater basin with highly diversified intensive agricultural production. J Contam Hydrol. 2013;151:140–154. doi: 10.1016/j.jconhyd.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Melvin S, Mabry J, Powers W, Kliebenstein J, Donham K, Hodne C. JA M, RF R. Iowa Concentrated Animal Feeding Operations Air Quality Study. Iowa City, Iowa: Environmental Health Sciences Research Center of the University of Iowa; 2002. Industry structure and trends in Iowa; pp. 18–34. [Google Scholar]
- Mellor AF, Cey EE. Using generalized additive mixed models to assess spatial, temporal, and hydrologic controls on bacteria and nitrate in a vulnerable agricultural aquifer. Journal of Contaminant Hydrology. 2015;182:104–116. doi: 10.1016/j.jconhyd.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Messier KP, Kane E, Bolich R, Serre ML. Nitrate variability in groundwater of North Carolina using monitoring and private well data models. Environmental Science and Technology. 2014;48(18):10804–10812. doi: 10.1021/es502725f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos C, Tzamtzis N, Liodakis S. Effects of an intensive hog farming operation on groundwater in east mediterranean (I): A study on electrical conductivity, as well as nitrogen and sulfur nutrients. Bulletin of Environmental Contamination and Toxicology. 2014;93(6):683–687. doi: 10.1007/s00128-014-1275-9. [DOI] [PubMed] [Google Scholar]
- Miller BA, Crumpton WG, Van Der Valk AG. Spatial distribution of historical wetland classes on the Des Moines Lobe, Iowa, Wetlands. 2009;29(4):1146–1152. [Google Scholar]
- Nolan BT, Hitt KJ. Vulnerability of shallow groundwater and drinking-water wells to nitrate in the United States. Environmental Science and Technology. 2006;40(24):7834–7840. doi: 10.1021/es060911u. [DOI] [PubMed] [Google Scholar]
- Puckett LJ. Identifying the major sources of nutrient water pollution. Environ Sci. & Technol. 1995;29(9):408–414. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. [accessed August 2015];R Foundation for Statistical Computing. 2006 Available at http:www.r-project.org.
- Ruddy BC, Lorenz DL, Mueller DK. [accessed November 2015];Scientific Investigations Report 2006–5012: County-Level Estimates of Nutrient Inputs to the Land Surface of the Conterminous United States, 1982–2001. 2006 available at http://pubs.usgs.gov/sir/2006/5012/
- Silverman BW. Density Estimation for Statistics and Data Analysis. London: Chapman & Hall; 1986. pp. 184–185. [Google Scholar]
- Tinker G. Interview by D. Wheeler [email] AFO Coordinator, Iowa Department of Natural Resources; 2015. Dec 17, [Google Scholar]
- Toetz D. Nitrate in ground and surface waters in the vicinity of a concentrated animal feeding operation. Archiv fur Hydrobiologie. 2006;166(1):67–77. [Google Scholar]
- USDA. [accessed May 2015];Animal Feeding Operations, United States Department of Agriculture. 2015 available at http://www.nrcs.usda.gov/wps/portal/nrcs/main/national/plantsanimals/livestock/afo.
- USEPA. Risk Assessment Evaluation for Concentrated Animal Feeding Operation. United States Environmental Protection Agency, Office of Research and Development, National Risk Management Research Laboratory. 2004 available at http://nepis.epa.gov/Exe/ZyPDF.cgi/901V0100.PDF?Dockey=901V0100.PDF. May 2004.
- Ward MH, deKok TM, Levallois P, Brender J, Gulis G, Nolan BT, VanDerslice J. Workgroup Report: Drinking-Water Nitrate and Health - Recent Findings and Research Needs. Environmental Health Perspectives. 2005;113(11):1607–1614. doi: 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH, Rusiecki JA, Lynch CF, Cantor KP. Nitrate in public water supplies and the risk of renal cell carcinoma. Cancer Causes Control. 2007;18:1141–1151. doi: 10.1007/s10552-007-9053-1. [DOI] [PubMed] [Google Scholar]
- Ward MH, Kilfoy BA, Weyer PK, Anderson KE, Folsom AR, Cerhan JR. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010;21(3):389–395. doi: 10.1097/EDE.0b013e3181d6201d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DC, Nolan BT, Flory AR, DellaValle CT, Ward MH. Modeling groundwater nitrate concentrations in private wells in Iowa. Science of the Total Environment. 2015;536:481–488. doi: 10.1016/j.scitotenv.2015.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. Generalized Additive Models: An Intro in R. Boca Raton: Chapman & Hall/CRC; 2006. [Google Scholar]