Abstract
Associative learning is essential for establishing appropriate responses to cause-effect relationships and effective behavioral adjustments to environmental changes. However, learned associations also promote maladaptive behavior such as uncontrollable drug seeking in addicts exposed to drug-associated stimuli. Here, we sought to identify behavioral characteristics that distinguish reward seeking produced by environmental stimuli conditioned to highly potent but non-addictive conventional reinforcers from reward seeking induced by stimuli conditioned to addictive drugs. Rats were trained to associate discriminative (i.e., contextual) stimuli (S+) with availability of cocaine, ethanol, palatable sweet solutions, or water during dehydration. Following extinction, response-reinstating effects of re-exposure to these stimuli were established in terms of magnitude and perseveration. Initially, the S+ produced strong reinstatement irrespective of association with conventional or drug reward. However, with repeated testing, S+-induced reward seeking decreased to extinction levels when motivated by the sweet solutions but perseverated when motivated by cocaine or ethanol. In rats placed on water restriction to induce a motivational constraint, the S+ supported perseverating reinstatement identical to that produced by an S+ conditioned to cocaine. The findings suggest that behavior guided by associations between environmental stimuli and drugs of abuse are characterized by perseverating, apparently highly extinction-resistant reward seeking, whereas behavior controlled by stimuli associated with conventional reward extinguishes rapidly in the absence of primary reinforcement. Reward seeking elicited by stimuli associated with natural reward can, however, become perseverative during physiological deprivation states. Possibly, perseverating drug seeking engages mechanisms overlapping with those that have evolved to promote alleviation of physiological deprivation to secure survival.
Keywords: addiction, alcohol, cocaine, conventional reinforcer, reinstatement
INTRODUCTION
Associative learning is an essential mechanism by which organisms learn about relationships between stimuli, to respond appropriately to cause-effect relationships, and to make appropriate behavioral adjustments in response to environmental changes. Learned associations can, however, also promote maladaptive behavior by uncontrollably dominating an organism’s behavioral repertoire as observed, for example, in drug addicts. Indeed, responses to drug-related stimuli such as drug desire (e.g., O'Brien et al., 1998; Van De Laar et al., 2004) or automatic drug-seeking behavior without distinct feelings of craving (Miller and Gold, 1994; Tiffany and Carter, 1998; Tiffany and Conklin, 2000) are a major factor in the chronically relapsing nature of drug addiction. In animals, environmental stimuli conditioned to drugs of abuse elicit reinstatement of drug seeking, with long-lasting effects that can even progressively increase with increasing abstinence duration, an effect referred to as the “incubation of craving” (e.g., Abdolahi et al., 2010; Bienkowski et al., 2004; Grimm et al., 2001; Shalev et al., 2001).
Although, conditioning factors have a firmly established role in the maintenance of drug compulsion, the learning processes that establish both adaptive goal-directed behavior and behavior that eventually develops into maladaptive drug seeking must be presumed to be identical. However, to the authors’ knowledge, no systematic comparative literature exists on long-term behavioral consequences of adaptive vs. drug-related maladaptive learning. A question addressed here therefore was what features characterize and distinguish reward seeking produced by environmental stimuli conditioned to highly potent but non-addictive conventional reinforcers vs. stimuli conditioned to highly addictive drugs of abuse. For this purpose, the effects of environmental stimuli associated with highly palatable sweet solutions were compared to those of stimuli associated with drug reward in terms of the magnitude and perseveration of reinstatement they produce. To establish the generality of these effects, the same stimuli were conditioned to qualitatively different non-drug reinforcers (i.e., sweet solutions differing in ingredients and caloric content) and drugs of abuse with different mechanisms of action (i.e., cocaine and ethanol). A complementary question was whether motivational constraints paralleling the state of drug deprivation experienced by rats during abstinence from drug self-administration modify reward seeking induced by stimuli conditioned to natural reward. To accomplish this, the salience of a natural reinforcer, water, was manipulated by inducing a physiological deprivation state via water restriction, and the reinstatement profile of stimuli conditioned to water availability was compared to that previously reported for stimuli conditioned to cocaine availability (Weiss et al., 2001).
MATERIAL AND METHODS
General Methods
Animals
Male Wistar rats (Charles River, Wilmington, MA; 200–250 g upon arrival) were housed 2–3 per cage in a temperature- and humidity-controlled vivarium on a 12 h/12 h light/dark cycle. Food and water were available ad libitum in the home cage. All procedures were conducted in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Intravenous and oral reinforcers
Cocaine hydrochloride (NIDA, Bethesda, MD) was dissolved in sterile physiological saline at a concentration of 0.25 mg/0.1 ml. Ethyl alcohol (EtOH) was diluted to a concentration of 10% (w/v) for self-administration. Non-drug reinforcers were sweetened condensed milk (SCM; Carnation, Nestlé, Vevey, Switzerland) diluted 1:3, and a 3% glucose/0.125% saccharin (w/v) solution (GSS). The sweet solution differed in caloric content (SCM: 1.4 Cal/ml; GSS: 0.1 Cal/ml). Oral reinforcers were prepared in dH2O.
Behavioural Testing Equipment
Rats were tested in operant conditioning chambers equipped with two retractable levers, white cue lights and a house light (Med Associates, St. Albans, VT). Intravenous and oral reinforcers were delivered using a syringe pump (Razel Scientific Instruments, Stamford, CT) located outside sound-attenuating cubicles housing the chambers. Cocaine was infused intravenously (IV) in a volume of 0.1 ml over 4 s. Oral reinforcers were dispensed at volumes of 0.1 ml into a drinking reservoir located in the centre of the chamber’s front panel. Auditory stimuli consisted of a 70 dB white noise and a 70 dB (7 kHz) intermittent tone (Sonalert; Med Associates, St. Albans, VT). Olfactory stimuli were banana or anise extracts (McCormick, Hunt Valley, MD) introduced 30 sec before session onset by depositing five drops of extract into the bedding of the chamber.
General behavioral procedures
Intravenous cocaine, oral ethanol, sweetened condensed milk (SCM), the glucose/saccharin solution (GSS), or water were established as reinforcers in different groups of rats on a FR1 schedule in sessions conducted 5 days/week for 14 days. Rats then completed a conditioning procedure in which responding at the active lever was differentially reinforced in the presence of discriminative stimuli (SD) signaling the availability (S+) or non-availability (S−) of reward. Conditioning sessions were initiated by extension of both levers and introduction of the respective SD and remained present throughout the session. Responding maintained by the drug or non-drug reinforcers then was extinguished in daily sessions conducted without SD presentation, and responses at the previously active lever had no scheduled consequences except for activation of the syringe pump motor. One day after reaching an extinction criterion of ≤ 5 responses/session for 3 consecutive days, rats were exposed to the respective reward S+ under extinction conditions every third day for a total of 4 sessions during which the number of responses at the previously active lever was recorded. To verify the selectivity of the S+ for reward seeking, rats were tested in the presence of the S− three days before the first S+ session.
Experimental Procedures
Cocaine vs. palatable sweet solution (SCM)
Rats were trained to self-administer COC (2 h/day) or SCM (40 min/day to prevent satiation by excessive SCM ingestion). Reinforced responses were accompanied by illumination of the cue light above the active lever (20 s), signaling a timeout (TO) period during which the lever remained inactive. After acquisition of COC- or SCM-reinforced responding, sessions continued under the SD-signaled differential reinforcement contingency. For this purpose, three 1 h (COC) or 20 min (SCM) sessions were conducted daily. The respective reinforcer was available during two S+-signaled (white noise) sessions, and unavailable during one S−-signaled (house light) session, conducted in random sequence and separated by 20-min intervals during which rats were removed from the chambers. During S− sessions, responses at the active lever were followed by a 20 s TO signal (intermittent beep; 1 s duration at 1 s intervals). Training continued under these conditions for 10 days (i.e., 20 reward and 10 non-reward sessions). Extinction and reinstatement sessions then were conducted under 2 different conditions to determine whether reinstatement session length is a factor in similarities or differences in reward seeking motivated by the COC vs. SCM-associated S+. In a first experiment (Exp 1), session length was held constant for each respective reinforcer across training, extinction and reinstatement phases at 60 min (COC) and 20 min (SCM). In a second experiment (Exp 2), extinction and reinstatement session length was held constant across reinforcers at 30 min.
Control for differences in olfactory stimulus information between SCM and COC
In contrast to IV COC, oral availability of SCM provides olfactory cues during conditioning that were absent during reinstatement tests in the design above. To ascertain that differential effects of the COC and SCM S+ on reinstatement are accounted for by drug vs. non-drug differences and not lack of the SCM olfactory stimulus, rats were provided with SCM as an olfactory cue in addition to the standard auditory S+ (white noise) by depositing 1 ml SCM into the bedding of the chamber before onset of reinstatement sessions. Additionally, extinction and reinstatement session length was extended to 60 min to match the standard COC reinstatement session length (Exp 1 above). SCM training and conditioning procedures were identical to those above.
Ethanol vs. palatable sweet solution (GSS)
The choice of ethanol served the purpose of ascertaining that any differences in reward seeking reflect differences in behavioral control by stimuli associated with drug vs. natural reward and not factors related to intravenous (cocaine) vs. oral (sweet solution) reward availability. Initially, responding maintained by GSS, a highly palatable sweet solution frequently employed to initiate oral EtOH self-administration (Walker and Koob, 2008), was established over 5 days in daily 30 min sessions. One group of rats then continued to self-administer GSS for 9 days. For the other group, daily sessions continued with 10% (w/v) EtOH added to the GSS solution and gradual elimination of sweeteners over the 9 d training period (Kufahl et al., 2011). EtOH or GSS sessions then continued under the SD-signaled differential reinforcement contingency identical to that for COC and SCM above, except that a compound auditory/olfactory stimulus context was used (S+: banana extract + white noise; S−: anise extract + a 7 kHz, 70 dB continuous tone). S+ and S− sessions were scheduled in random sequence for a total of 10 S+ and 10 S− sessions. EtOH- and GSS-maintained responding then was extinguished in daily 30 min sessions, followed by reinstatement testing.
Reinstatement by stimuli conditioned to natural reward under deprivation conditions
Rats were fluid-restricted and received 2 h of daily access to water via self-administration on an FR1 schedule (0.1 ml/response), followed by 1 h of unlimited access to water from a bottle in their home cage. During weekends (Friday 5:00 pm to Sunday 5:00 pm), water was available ad libitum. The mild water restriction regimen was continued until completion of the experiment to generate a motivational constraint resembling the drug deprivation experienced by rats with histories of cocaine or EtOH self-administration. After acquisition of water-maintained responding, rats underwent conditioning and extinction procedures identical to those for COC self-administering rats (Exp 1) with minor modifications in the stimuli signaling water availability (S+: intermittent tone) vs. non-availability (S−: white cue light). Responses at the active lever were followed by a signaled (house light or white noise) 20 s TO period during which the lever remained inactive. These stimulus combinations were selected to match those of an earlier experiment that reported long-lasting stimulus-controlled reinstatement of cocaine seeking (Weiss et al., 2001). The experimental design and sequence of reinstatement testing at 3-day intervals were identical to those in COC self-administering rats above, except that the test phase was extended to also match the conditions in (Weiss et al., 2001). S+ effects were examined in ten sessions (days 4–28, 34 post-extinction) and S− effects in two sessions (days 1, 31 post-extinction).
Statistical analyses
Differences between reward (cocaine, SCM, ethanol, GSS, or water) and non-reward responding during the training phase were analyzed by paired t-test. Differences between the number of extinction days (were analyzed by unpaired t-test). Differences between reinstatement induced by the cocaine-, SCM-, ethanol-, GSS-, and water-related S+ were analyzed by one-way repeated-measures or mixed-factorial analysis of variance (ANOVA), followed by pairwise comparisons (simple effects) or the Protected Least Significant Difference (PLSD) post hoc test to compare differences between each reinstatement test.
RESULTS
Cocaine vs. conventional reward (SCM)
Experiment 1 (Constant session length across experimental phases)
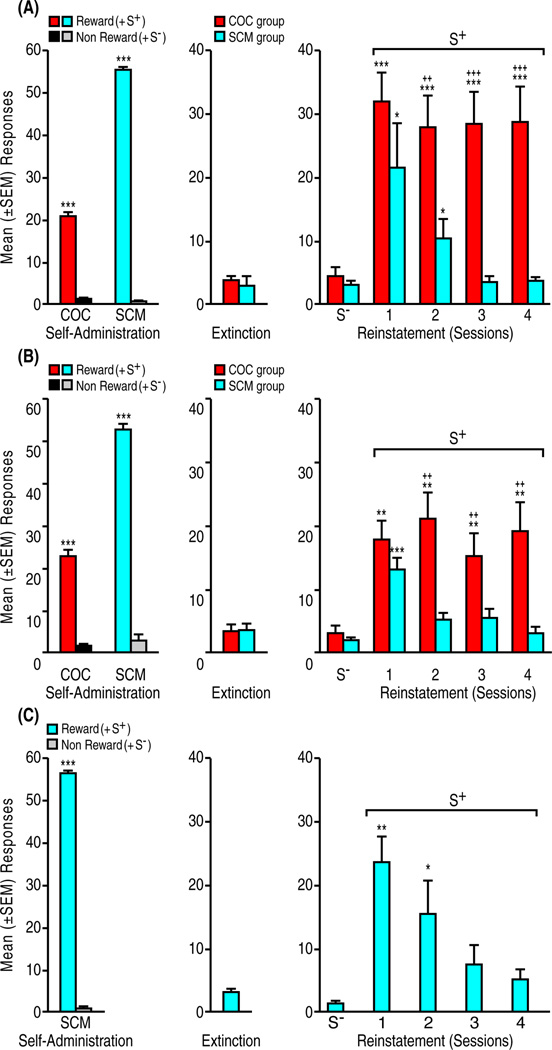
Both COC (n = 8) and SCM (n = 8) supported stable responding in the S+-signaled reward condition, whereas responding declined to negligible levels under S−-signaled non-reward conditions (COC: t7 = 10.3, p < 0.001; SCM: t7 = 27.6, p < 0.001; pooled across the final 3 sessions; Fig. 1A, left panel). Rats reached the extinction criterion within a mean (± SEM) of 9.0 ± 2.0 (COC) and 6.0 ± 1.3 (SCM) days (t14 = 1.2, NS; Fig. 1A, center panel). In subsequent reinstatement tests, the S+ produced significant reward seeking but with a different profile of effects depending on its association with COC vs. SCM reward (COC vs. SCM: F1,14 = 14.9, p < 0.01; reinstatement: F3,42 = 5.9, p < 0.01; reward × reinstatement interaction: F3,42 = 2.8, p < 0.05; Fig. 1A, right panel). S+-induced COC seeking differed significantly from extinction levels and remained stable with repeated testing (p < 0.001). S+-induced SCM seeking did not differ statistically from S+-induced COC seeking during the first reinstatement test (PLSD post hoc analysis), but subsequently decreased, remaining significant from extinction only during the second test (p < 0.05), with a further decline toward extinction levels over the remaining tests (pairwise comparisons following ANOVA; COC: F5,35 = 23.0, p < 0.001; SCM: F5,35 = 7.1, p < 0.001).
Figure 1.
Differential perseveration of reinstatement by stimuli conditioned to cocaine (COC) vs. natural reward (SCM). (A) Session lengths constant across experimental phases for the respective reinforcer (COC = 60 min; SCM 20 min). (B) Reinstatement session lengths modified to be constant across reinforcers (COC = 30 min; SCM 30 min). (C) Effects of SCM stimulus information (“compound” SD consisting of the auditory S+ plus the SCM olfactory cue) on reinstatement. Self-administration (left panels A – C): Mean (±SEM) responses averaged across the final three self-administration/conditioning sessions. Responding was differentially reinforced in the presence of discriminative (i.e., occasion setting contextual) stimuli signaling the availability (S+) or nonavailability (S−) of COC or SCM. ***p < 0.001 vs. non-reward and S− conditions. Extinction (center panels A – C): Mean (±SEM) non-reinforced responses averaged across the final three extinction sessions. Reinstatement (right panels A – C): Effects of the non-reward S− tested one day after the final extinction session, followed by effects of the reward-predictive S+ across four reinstatement sessions. ***p < 0.001, **p < 0.01, *p < 0.05, vs. corresponding EXT and S− tests; +++p < 0.001, ++p < 0.01, vs. SCM S+.
Experiment 2 (Constant session length across reinforcers)
Here, session length was modified after completion of the extinction phase for both the COC and SCM group to provide for identical duration of reinstatement tests (i.e., 30 min). Both COC (n = 8) and SCM (n = 9) maintained stable responding under S+-signaled reward conditions and responding decreased to negligible levels in the S−-signaled non-reward condition (COC: t7 = 13.7, p < 0.001; SCM: t8 = 17.7, p < 0.001; Fig. 1B, left panel). There were no differences between the COC (10.2 ± 1.8) and SCM (7.0 ± 1.0) groups in the number of days to extinction criterion (t15 = 1.7, NS; Fig. 1B, center panel). Reinstatement tests again revealed a reward-dependent differential perseveration of S+-induced behavior (COC vs. SCM: F1,15 = 6.9, p < 0.01; reinstatement: F3,45 = 3.5, p < 0.05; reward × reinstatement interaction: F3,45 = 4.8, p < 0.01; Fig. 1B, right panel). This effect was characterized by statistically equivalent S+-induced recovery of responding in the COC and SCM groups (p < 0.001) during the first test (PLSD post hoc test: NS), perseveration of COC S+-induced reinstatement across repeated tests (p < 0.001), and failure of the SCM S+ to elicit reinstatement beyond the first test (pairwise comparisons following ANOVA; COC: F5,35 = 12.4, p < 0.001; SCM: F5,40 = 14.3, p < 0.001).
Effects of SCM olfactory stimulus information during reinstatement tests
Like the SCM groups above, rats (n = 7) showed high levels of responding during SCM availability and negligible responding during SCM non-availability (t6 = 77.1, p < 0.001; Fig. 1C, left panel). Following extinction (11.0 ± 2.0 days to criterion), re-exposure to the SCM S+ including SCM olfactory stimulus information, but the not S−, produced reinstatement that was significant during the first (p < 0.01) and second (p < 0.05) test (pairwise comparisons following ANOVA; F5,30 = 10.0, p < 0.001, Fig. 1C, right panel). As in the SCM groups of experiments 1 and 2, S+-induced reinstatement declined over repeated tests, reaching extinction levels by the third session.
EtOH vs. conventional reward (GSS)
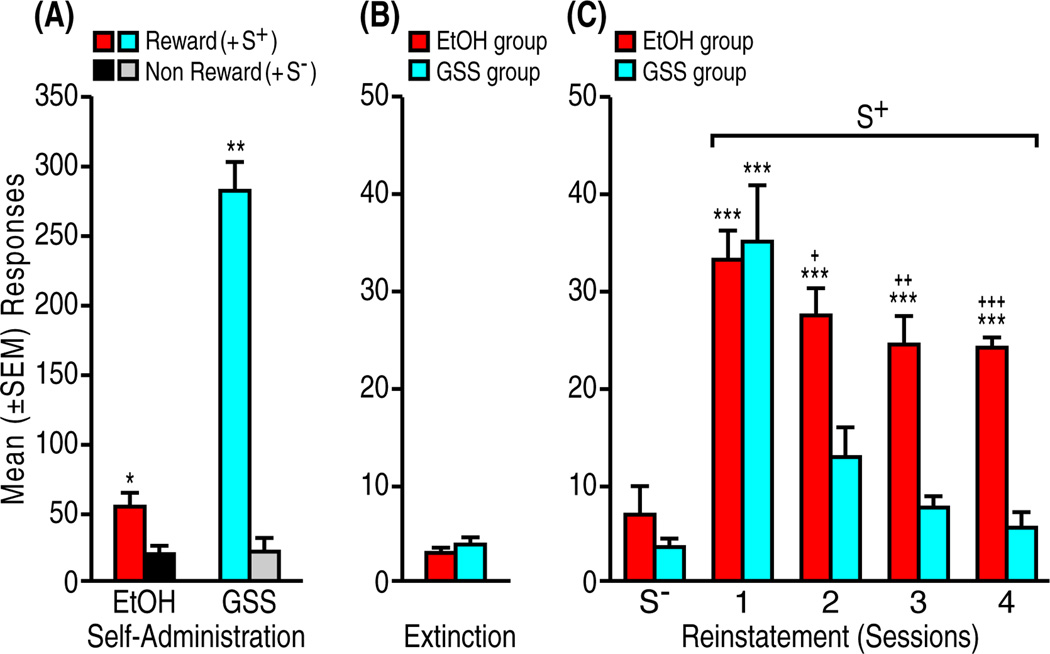
Rats reliably self-administered EtOH (n = 5) and GSS (n = 4) at the end of the conditioning phase with minimal responding under non-reward conditions (EtOH: t4 = 3.03, p < 0.05; GSS: t3 = 10.7, p < 0.01) and reached criterion during subsequent extinction sessions without differences between groups (EtOH: 21.6 ± 0.8 days, GSS: 18.0 ± 2 days; t3 = 10.7, NS; Fig. 2A, B). As in the case of COC vs. SCM, a reward-dependent differential reinstatement profile was observed (EtOH vs. GSS: F1,7 = 20.3, p < 0.001; repeated reinstatement: F3,21 = 21.7, p < 0.001; reward × reinstatement interaction: F3,21 = 6.1, p < 0.01; Fig. 2C). The EtOH S+ produced significant responding that remained stable over the four tests (p < 0.001), whereas reinstatement induced by the GSS S+ was significant (p < 0.001) only during the first test and decreased to extinction levels over subsequent tests (pairwise comparisons following ANOVA; EtOH: F5,20 = 29.1, p < 0.001; GSS: F5,15 = 18.6, p < 0.001).
Figure 2.
Differential perseveration of reinstatement by stimuli conditioned to EtOH vs. natural reward (GSS). (A) Self-administration: Mean (±SEM) responses during reinforcer availability (Reward + S+) vs. nonavailability (Non Reward + S−) averaged across the final three self-administration/conditioning sessions. *p < 0.05, **p < 0.01, vs. non-reward. (B) Extinction: Non-reinforced responses averaged across the final three extinction sessions. (C) Reinstatement: Effects of S+ and S− presentation on reinstatement in tests conducted according the same sequence as in Figure 1. ***p < 0.001, **p < 0.01, vs. extinction and S−; +p < 0.05, ++p < 0.01, +++p < 0.001, vs. GSS S+.
Perseveration of reinstatement motivated by natural reward under deprivation conditions
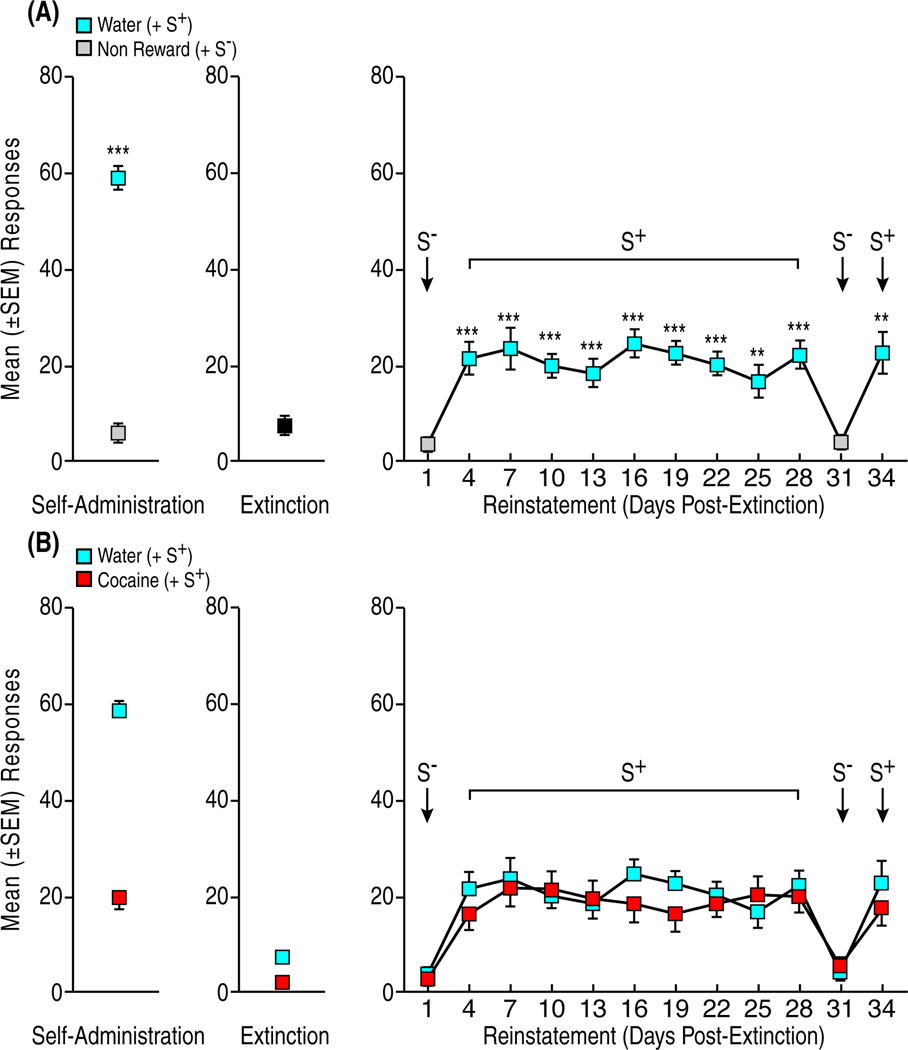
Rats (n = 12) showed stable water-reinforced responding during S+-signaled reward sessions at the end of the conditioning phase, with only minimal responding in the non-reward condition (pooled across the final 3 days: t11 = 20.6, p < 0.001). Rats reached the extinction criterion within 7.5 ± 0.9 days (Fig. 3A, left and center panels). The water S+ produced significant (p < 0.001) reinstatement that persisted throughout the eleven S+ tests (pairwise comparisons following ANOVA; F12,132 = 9.8, p < 0.001). The S− either failed to elicit (day 1) or reversed reinstatement (day 31) to extinction levels (Fig. 3A, right panel). This profile of S+ and S−-controlled water seeking resembled in all aspects the profile of long-lasting S+ and S−-controlled cocaine seeking (Fig. 3B) in an earlier report (Weiss et al., 2001).
Figure 3.
Perseveration of reinstatement motivated by natural reward under deprivation conditions. (A) Mean (±SEM) responses during S+-signaled water availability and S−-signaled water non-availability across the final three sessions (Self-Administration). ***p < 0.001, vs. non-reward. Following extinction (center panel), exposure to the water-predictive S+ produced robust reinstatement that remained stable over multiple tests (right panel). Responding in the presence of the S− remained at (Day 1) or returned to (Day 31) extinction levels. ***p < 0.001, **p < 0.01, vs. extinction and S− (B) Superimposition of the data in (A) over S+/S−-controlled cocaine seeking in an earlier study (Weiss et al., 2001) that employed the same stimuli and training protocols as in (A). Data obtained from (Weiss et al., 2001) with permission.
DISCUSSION
All reward-predictive environmental stimuli (S+) produced strong recovery of responding regardless of whether they had been conditioned to conventional or drug reward. With repeated testing, however, behavior induced by these stimuli decreased to levels indistinguishable from extinction performance when motivated by conventional reinforcers with high hedonic value, but perseverated at undiminished levels when motivated by cocaine or ethanol. This differential reinstatement profile was highly reliable in that it remained unchanged over several replications, two different classes of commonly abused drugs, sweet solutions differing in caloric content, and modification of test conditions including session length, as well as olfactory, visual and auditory cue information.
The results suggest that, in contrast to behavior guided by associations between environmental stimuli and conventional reward that follows established rules of learning and reinforcement (i.e., extinction of the conditioned behavior as a result of repeated exposure to the reward predictive S+ absent the primary reinforcer), learning that links reward-predictive stimuli with drugs of abuse produces compulsive-like behavior reflected by perseveration of drug seeking despite continued non-reinforced exposure to these stimuli. Before accepting these conclusions, alternative explanations must be examined. First, oral availability of the sweet solutions provided olfactory cues during self-administration, possibly adding a salient stimulus dimension to the S+ context not present during intravenous self-administration of cocaine. The absence of this stimulus dimension during reinstatement testing may have accelerated extinction learning. Manipulations introduced to address this issue, rule out this interpretation. An S+ conditioned to ethanol, an oral reinforcer with a strong olfactory stimulus dimension, produced a reinstatement profile distinctly different from that of the S+ conditioned to the sweet solutions and identical to that produced by the cocaine S+. Moreover, SCM olfactory cue information, introduced in experiment 2 (Fig. 1C) to identify effects of this stimulus dimension on reinstatement, neither accelerated nor retarded the rapid the loss of responsiveness to the S+. Therefore, the findings cannot be explained by differences in conditioning due to the presence vs. absence of extraneous olfactory stimulus information. Second, differences in the reinforcing magnitude between the drug and non-drug reinforcers may have resulted in differential “strength” of conditioning and, therefore, differences in the efficacy of the respective stimuli to elicit and maintain behavior. However, this interpretation is not compelling. As documented in a classic study of SCM reinforcement (Hodos, 1961) and recent data with GSS (unpublished observations), ad libitum-fed rats show progressive ratio schedule breakpoints for these sweet solution reinforcers that are considerably higher (~60) than those produced by ethanol (~20; e.g., Brown et al., 1998; Ciccocioppo et al., 2003). Thus, although the ethanol S+ maintained perseverating reinstatement, ethanol is a less potent primary reinforcer than the sweet solutions. Lastly, levels of reinstatement motivated by the two drugs of abuse and sweet solutions were statistically identical on the first test day, arguing further against differences in reinforcing magnitude as an alternative explanation for the time-dependent differences in drug vs. conventional reward seeking. The results, therefore, support the conclusion that perseveration of reinstatement or “reward craving” resulting from reward-environment associations is a phenomenon linked preferentially to drugs of abuse and independent of the initial primary reinforcing strength of the substance maintaining behavior during self-administration.
No direct evidence is available concerning the neurobehavioral mechanisms that regulate the eventual extinction of behavior motivated by stimuli conditioned to conventional reward but fail to do so in the case of drug reward. Distinct populations of nucleus accumbens (NAcc) neurons differentially encode information about natural rewards vs. cocaine or ethanol (Carelli et al., 2000; Carelli and Wondolowski, 2006; Robinson and Carelli, 2008) and drugs of abuse activate NAcc neurons largely separate from those engaged during goal-directed behavior associated with natural reward (Cameron and Carelli, 2012). By extension, the response-reinstating effects of stimuli associated with drug vs. natural reward may be mediated by distinct sets of neurons within the NAcc. However, if this were the case, it remains unclear by what process these neural subpopulations come to promote perseverating behavior when motivated by drug cues as opposed to regulating the rapid cessation of behavior when motivated by natural reward. One possibility is that neurons mediating drug seeking are neuroadaptively altered by cocaine and ethanol, preventing the “unlearning” of drug-cue associations, a process that does not occur with conventional rewards. In support of this possibility, cocaine induces metaplasticity in the NAcc (Moussawi et al., 2009), indicative of a deficit in the ability to induce plasticity in cortico-accumbens circuitry that is essential for learning of new or alternative behavior (e.g., Kalivas and O'Brien, 2008; Moussawi et al., 2009). Another process possibly accounting for the findings is that drug seeking, but not behavior motivated by the sweet solutions, transitioned from goal-directed to habitual (i.e., action-outcome independent) behavior, resulting in perseveration of this behavior in a non-goal oriented manner (e.g., Belin and Everitt, 2008; Corbit et al., 2012). This transition is accompanied by a shift in neural control from the Nacc to the dorsolateral striatum (DLS) in animals (Belin and Everitt, 2008; Corbit et al., 2012). Corresponding findings have been described in man with greater cue-induced activation of the ventral striatum in light social drinkers and greater cue-induced activation of the dorsal striatum in heavy drinkers (Vollstadt-Klein et al., 2010). Nonetheless, this account awaits confirmation by testing the sensitivity of perseverating reinstatement to DLS lesions (e.g., Murray et al., 2014) and resistance to reward devaluation.
Despite the considerable hedonic value of the sweet solutions, stimuli associated with their availability failed to elicit perseverating reward seeking. However, when conditioned to the availability of water, a non-drug substance with little hedonic value, the same stimuli supported a drug seeking-like perseverating reinstatement profile in rats placed on a water restriction regimen. This manipulation was introduced based on the conceptualization that termination of access to drugs following daily self-administration results in a deprivation state that increases demand for the drug reinforcer, and that this altered motivational state may be reproducible in the case of conventional reward by induction of a physiological need state. The results confirm that stimuli predictive of the opportunity to alleviate a physiological deprivation state, like stimuli predicting drug availability during drug deprivation, elicit a perseverating profile of seeking behavior. However, it is not clear whether both behaviors are controlled by a common process Perseverating drug seeking likely depends on mechanisms such as those discussed above. On the other hand, seeking behavior motivated by natural reward under deprivation conditions may engage innate mechanisms that have evolved to promote survival by perpetuating responsiveness to reward-predictive signals under conditions in which securing the reward is essential to the survival of the organism as documented in a classical study showing that high concentrations of salt that are normally avoided become palatable to salt-deprived rats (Berridge et al., 1984). Whether drug seeking engages or usurps such mechanism to promote perseverating responsiveness to drug cues and, by inference, vulnerability to relapse is an intriguing hypothesis for future testing.
The perseveration of drug seeking at undiminished levels over repeated tests conducted in close succession described here is distinct from behavior obtained in tests of reward seeking following long and increasing periods of abstinence, which is characterized by time-dependent increases in drug seeking described in both animals (Bienkowski et al., 2004; Grimm et al., 2001) and man (Bedi et al., 2011; Li et al., 2014; Wang et al., 2013) and known as the “incubation of craving.” The present “perseveration of craving” – like the incubation effect (e.g., Pickens et al., 2011) – generalized across drugs of abuse. However, in contrast to the incubation effect that generalizes also to conventional sweet solution reinforcers (Aoyama et al., 2014; Grimm et al., 2005; Grimm et al., 2003), the perseveration of reward seeking was distinctly selective for drug-directed behavior. The experimental conditions under which reward seeking is measured in the two models likely capture different aspects of reward craving and, by extension, the drug relapse process (Marchant et al., 2013). The incubation effect is typically observed when subjects are reintroduced to reward-paired environments after periods of abstinence during which such cues remain absent. The perseveration of reward craving described here, on the other hand, appears to reflect control of behavior by drug-associated stimulus environments that is extinction-resistant despite frequent exposure to these environments under extinction conditions, a condition typically experienced by addicts during ongoing drug use or attempts to abstain while remaining in their drug-associated settings.
Overall, the findings reveal that, under the presently employed contingencies, environmental stimuli conditioned to cocaine or ethanol produce a distinctly different reward seeking profile than stimuli conditioned to conventional reinforcers with high hedonic value. These differential effects were highly reliable across a range of experimental manipulations and reflect the compulsive character of addiction with drug seeking that appears impervious to extinction versus the expected normal decay of non-reinforced behavior in the case of natural reward. These observations are consistent with, and extend previous data showing that rats that had been given a single life-time opportunity to self-administer cocaine still exhibited cocaine seeking one year later whereas SCM seeking was no longer observed three months after a single opportunity to self-administer the sweet solution (Ciccocioppo et al., 2004). The control of reward seeking by stimulus contexts conditioned to natural reward was, however, not inflexibly distinct from that exerted by drug-related environments but attained perseverative character under conditions that, in terms of behavioral economics, presumably reduced the elasticity of demand for the reinforcer. The procedures employed to establish the differential persistence of behavior that characterizes normal vs. drug- and deprivation-induced reward seeking provide an effective tool to increase understanding of what distinguishes the control of these behaviors at the neurocircuitry and molecular level, and whether perseverating drug seeking engages mechanisms overlapping with those that evolved to promote survival in the presence of physiological deprivations states or is controlled by distinct processes that, nonetheless, result in the same behavioral outcome.
Acknowledgments
This is publication number 21433 from The Scripps Research Institute. The authors thank O. Ben-Shahar, N. Stuempfig, J. Simms, C. Lorentz, K. Pedersen, and T. Kerr for behavioral testing and data collection. M. Arends provided editorial assistance during the preparation of this manuscript. Supported by NIH/NIDA: DA07348, DA08467 (F.W.), DA033344 (R.M-F.), and NIH/NIAAA: AA10531, AA14351, AA018010 (F.W.).
Footnotes
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
AUTHOR CONTRIBUTIONS
R.M.-F. and F.W. were responsible for the study concept and design. R.M.-F performed the experiments and statistical analyses, and interpreted the findings. R.M.-F. and F.W. wrote the manuscript. All authors critically reviewed the content and approved the final version for publication.
REFERENCES
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Barnes J, Grimm JW. Incubation of saccharin craving and within-session changes in responding for a cue previously associated with saccharin. Appetite. 2014;72:114–122. doi: 10.1016/j.appet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98:652–660. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, Bogucka-Bonikowska A, Kostowski W. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Brown G, Jackson A, Stephens DN. Effects of repeated withdrawal from chronic ethanol on oral self-administration of ethanol on a progressive ratio schedule. Behav Pharmacol. 1998;9:149–161. [PubMed] [Google Scholar]
- Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci. 2012;35:940–951. doi: 10.1111/j.1460-9568.2012.08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus "natural" (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Anatomic distribution of reinforcer selective cell firing in the core and shell of the nucleus accumbens. Synapse. 2006;59:69–73. doi: 10.1002/syn.20217. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat Neurosci. 2004;7:495–496. doi: 10.1038/nn1219. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36:2762–2773. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, Ma MY, Xue MM, Luo YX, Yang FD, Bao YP, Shi J, Sun HQ, Lu L. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2014 doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, Gold MS. Dissociation of "conscious desire" (craving) from and relapse in alcohol and cocaine dependence. Ann Clin Psychiatry. 1994;6:99–106. doi: 10.3109/10401239409148988. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Dilleen R, Pelloux Y, Economidou D, Dalley JW, Belin D, Everitt BJ. Increased impulsivity retards the transition to dorsolateral striatal dopamine control of cocaine seeking. Biol Psychiatry. 2014;76:15–22. doi: 10.1016/j.biopsych.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Carelli RM. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur J Neurosci. 2008;28:1887–1894. doi: 10.1111/j.1460-9568.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Van De Laar MC, Licht R, Franken IH, Hendriks VM. Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology (Berl) 2004;177:121–129. doi: 10.1007/s00213-004-1928-1. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]