Abstract
Background
Motivational enhancement (ME) shows promise as a means of increasing adherence to CPAP for OSA.
Methods
We performed an open-label, parallel-arm, randomized controlled trial of CPAP only or CPAP + ME, recruiting individuals 45 to 75 years with moderate or severe OSA without marked sleepiness and with either established cardiovascular disease (CVD) or at risk for CVD. All participants received standardized CPAP support from a sleep technologist; those randomly assigned to CPAP + ME also received standardized ME delivered by a psychologist during two appointments and six phone calls over 32 weeks. Mixed-effect models with subject-specific intercepts and slopes were fitted to compare objective CPAP adherence between arms, adjusting for follow-up duration, randomization factors, and device manufacturer. All analyses were intention-to-treat.
Results
Overall, 83 participants (n = 42 CPAP only; n = 41 CPAP + ME) contributed 14,273 nights of data for 6 months. Participants were predominantly male (67%) and had a mean ± SD age of 63.9 ± 7.4 years, a BMI of 31.1 ± 5.2 kg/m2, and an apnea-hypopnea index of 26.2 ± 12.9 events/h. In our fully adjusted model, average nightly adherence for 6 months was 99.0 min/night higher with CPAP + ME compared with CPAP only (P = .003; primary analysis). A subset of 52 participants remained in the study for 12 months; modeling these data yielded a consistent difference in adherence between arms of 97 min/night (P = .006) favoring CPAP + ME.
Conclusions
ME delivered during brief appointments and phone calls resulted in a clinically significant increase in CPAP adherence. This strategy may represent a feasible approach for optimizing management of OSA.
Trial Registry
ClinicalTrials.gov; No.: NCT01261390; URL: www.clinicaltrials.gov.
Key Words: adherence, behavioral therapy, CPAP, OSA, randomized controlled trial
Abbreviations: AASM, American Academy of Sleep Medicine; AHI, apnea-hypopnea index; BestAIR, Best Apnea Interventions in Research study; BIDMC, Beth Israel Deaconess Medical Center; BWH, Brigham and Women’s Hospital; CVD, cardiovascular disease; ESS, Epworth Sleepiness Scale; ME, motivational enhancement; PHQ-8, Patient Health Questionnaire 8-item depression scale; SAVE, Sleep Apnea cardioVascular Endpoints study; SEMSA, Self Efficacy Measure for Sleep Apnea; WHI, Women’s Health Initiative
CPAP is currently the standard intervention for moderate or severe OSA; however, effectiveness is limited by poor acceptance and adherence. The literature suggests that at least 50% of patients use CPAP for less than 4.0 h/night.1 There appears to be a dose-response relationship between CPAP adherence and improvements in health and quality of life, with studies indicating that substantial improvements in sleepiness, memory, functional status, and blood pressure, as well as reduced rates of cardiovascular mortality, are most obvious in those using CPAP more than 5.5 h/night.2, 3, 4, 5, 6
Participants enrolled in research studies—particularly randomized trials—are usually provided close follow-up support intended to maximize adherence, but even under such conditions, CPAP adherence is often modest. This may be particularly problematic in patients with cardiovascular comorbidity; for example in the ongoing Sleep Apnea cardioVascular Endpoints (SAVE) trial, average CPAP adherence after 6 and 12 months was 3.8 and 3.3 h/night, respectively.7 Adjunct behavioral therapy such as motivational enhancement (ME) shows promise as a means of maximizing CPAP adherence8, 9, 10, 11, 12; however, it has not yet entered standard clinical practice largely because of the absence of rigorous collected evidence on its efficacy.
The Best Apnea Interventions for Research (BestAIR) study was a parallel-arm randomized controlled trial designed to investigate the effect of CPAP on blood pressure. A secondary, prespecified objective was to compare adherence between participants with OSA and cardiovascular comorbidity randomly assigned to CPAP alone versus CPAP + ME to determine the impact of this additional therapy on adherence. We hypothesized greater CPAP adherence for 6 months among those randomly assigned to CPAP + ME compared with CPAP only. We also aimed to explore adherence for 12 months in a subset of participants, investigate the change in adherence over time, assess sleep duration as a potential mediator of the impact of ME on adherence, and investigate potential effect modification by baseline sleepiness, self-efficacy, self-reported depression and insomnia symptoms, recruitment site, and cardiovascular disease (CVD) status.
Methods
Methodology of the BestAIR study has been published.13, 14 Approval for the study was obtained from the institutional review boards at Brigham and Women’s Hospital (BWH) (Partners Human Research Committee, protocol 2010P002671), Beth Israel Deaconess Medical Center (BIDMC), and the Joslin Diabetes Center (assessed jointly at the BIDMC Committee of Clinical Investigations, protocol 2011P000012). The study was registered at clinicaltrials.gov (NCT01261390). All participants gave written informed consent to participate.
Study Design
This study was an open-label, parallel-arm, randomized controlled trial of CPAP only or CPAP + ME, with follow-up duration of up to 12 months.
Entry Criteria, Run-In Phase, and Randomization
We recruited subjects from outpatient cardiology, diabetes, and sleep clinics who met study eligibility criteria and were willing to be randomly assigned to alternative study arms. Subjects were recruited either (1) face-to-face, which involved screening the electronic medical records for patients with upcoming clinic appointments, obtaining physician approval, and then making an in-person approach by a research assistant, or (2) through mailings, which involved screening the electronic medical records of patients at nine local cardiology clinics, obtaining physician approval, and mailing letters of invitation to potential participants.13
Subjects meeting the following inclusion criteria were recruited: OSA (apnea hypopnea index [AHI] 4%, ≥ 10 events/h; or AHI 3%, ≥ 15 events/h); 45 to 75 years with established CVD or cardiometabolic disease (established coronary artery disease [≥ 70% stenosis in at least one major coronary artery], prior myocardial infarction, coronary artery revascularization procedure, ischemic stroke, or diabetes), or 55 to 75 years with at least three CVD risk factors (male sex, BMI ≥ 30 kg/m2, hypertension, dyslipidemia, and ≥ 10 pack-years of smoking). Exclusion criteria were a cardiovascular event < 4 months before enrollment, prior CPAP, Epworth Sleepiness Scale (ESS) score > 14 of 24, drowsy driving within 2 years, commercial driving, or an uncontrolled medical condition (including central sleep apnea, heart failure, uncontrolled hypertension, severe hypoxemia, anemia, and renal insufficiency). Drowsy driving, commercial driving, and a moderate or high ESS score were used for eligibility to enhance safety considering that the BestAIR study included two untreated control conditions, with intervention lasting up to 1 year (see subsequent section).
Eligible participants entered a run-in phase before randomization, consisting of 14 days wearing a nasal CPAP mask during sleep (without a CPAP device). Participants who reported using the mask during the majority of the run-in and who were willing to continue using the mask were eligible for randomization to one of four study arms (two control conditions, two treatment conditions): conservative medical therapy, sham CPAP, active CPAP, or active CPAP + ME (Fig 1). Analyses in this paper compare the active CPAP and CPAP + ME arms only. Randomization took place in a 1:1:1:1 ratio with a block size of 4, based on three stratification factors: diagnostic study (full night or split night with titration), site (BWH, BIDMC, or Joslin), CVD status (established or risk factors). Randomization was performed using a data-entry system linked to an off-site server holding the sequences. Participants randomly assigned to a CPAP group immediately underwent secondary randomization to use a device by one of two manufacturers (Philips Respironics or ResMed), using a randomization sequence with a block size of 2.
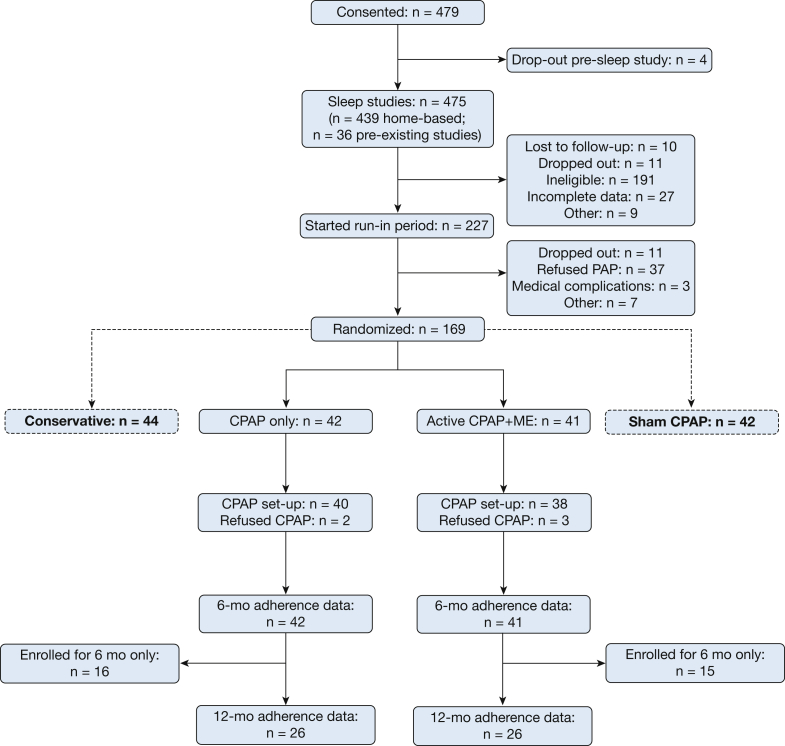
Figure 1.
Trial flow-chart. As part of the larger Best Apnea Interventions in Research trial, 169 participants were randomly assigned to conservative therapy, sham CPAP, CPAP only, or CPAP + ME. Participants in the CPAP-only and CPAP + ME arms were included in the current analyses (n = 83). Any nights of non-CPAP usage, including all nights for participants who refused CPAP set-up, were included in the data set as 0.0 hours; hence, data completeness was 100%. Primary outcome was adherence at 6 months; 12-month adherence data were included in a secondary analysis. ME = motivational enhancement.
Sleep Studies and CPAP Administration
Sleep studies took place either through normal clinical channels for those recruited from sleep clinics (level 1 polysomnography or level 3 home sleep tests), or administered by the investigators for those recruited from cardiology or diabetes clinics (using either Embletta Gold or Embletta X100, Embla). All studies were scored by a single registered polysomnographic technologist using the American Academy of Sleep Medicine (AASM) alternative hypopnea criteria when arousal data were available, or the AASM-recommended criteria when electroencephalographic data were not recorded.15
Participants were instructed on the use of CPAP during a 1-hour appointment with a sleep technologist, which followed a written manual of procedures and a standardized checklist. Unless an in-laboratory CPAP titration had been performed, participants used an autoadjusting device for a minimum of 5 days with the 90th or 95th percentile pressure used for ongoing fixed CPAP depending on the device manufacturer (Philips Respironics and ResMed devices report the 90th and 95th percentile pressures, respectively). CPAP devices used were the REMStar Auto (Philips Respironics) or the S9 Autoset (ResMed).
The original trial design included follow-up for 12 months; however, subjects randomly assigned after January 2013 were restricted to 6 months of follow-up.13 Our primary analysis of CPAP adherence therefore took place for 6 months, rather than 12. During follow-up, all participants were offered 30-minute appointments with a sleep technologist 1 week, 1 month, 3 months, 6 months, and (where applicable) 9 months after randomization, focused on OSA and CPAP education and technical troubleshooting.
Subjects randomly assigned to the CPAP-only arm underwent sleep studies and CPAP administration as described. Those randomly assigned to CPAP + ME underwent the intervention in addition to the procedures performed in the CPAP-only arm.
Intervention Arm
ME is a behavioral intervention devised on the principles of motivational interviewing.16, 17 The premise of the therapy is to honor the natural ambivalence that accompanies any change to behavior and to approach the patient in a thoughtful and empathetic manner that elicits critical thought to maximize behavior change. The ME protocol was designed by Aloia et al8, 18 and has been published previously. The overall goal of each ME session was to resolve the subjects’ ambivalence toward establishing consistent CPAP usage patterns, and increase the subjects’ confidence toward using CPAP regularly. The psychologist delivering the intervention (C. T.) aimed to maintain a collaborative—rather than educational—style of interaction with each subject. Each session involved a discussion regarding the subject’s readiness to begin CPAP, the subject’s understanding of the health risks associated with untreated OSA, and the extent to which the subject believed that consistent CPAP use could resolve these risks. Each subject was encouraged to set concrete goals regarding their future CPAP use, and identify rewards that they would provide themselves when those goals were achieved.
Doctor Aloia trained the psychologist (C. T.) who delivered the therapy for this trial. Training consisted of a day-long training session, a written manual, instructional videos, and several follow-up phone calls. ME was delivered during 1-hour in-person sessions at baseline and week 1, which included an educational video, and during phone calls of 10 to 30 minutes with the same psychologist at weeks 3, 4, 8, 12, 20, and 32. In-person sessions were audio-recorded, to allow independent assessment of fidelity to the intervention framework.
Data Collection
All CPAP devices were equipped with a modem to transmit nightly adherence data to secure servers, and thus data collection was not contingent on attendance at appointments. Any nights of nonusage were included in the dataset as 0.0 h/night, including for participants who stopped using their CPAP before trial completion. The 6- and 12-month time points used for analysis coincided with dates of attendance of follow-up visits. In cases of nonattendance, dummy dates were created 180 days or 365 days postrandomization to determine the number of nights of adherence data for each participant.
Questionnaires (ESS,19 Self-Efficacy Measure for Sleep Apnea [SEMSA],20 Patient Health Questionnaire 8-item Depression Scale [PHQ-8],21 and Women’s Health Initiative [WHI] Insomnia Rating Scale22) were administered before randomization. Usual available sleep time was assessed with the questions, “How many hours of sleep do you usually get per night on weekdays/workdays?” and “ . . . on weekends/days off?,” assuming 5 workdays and 2 days off per week. Usual time awake during the night was assessed with the questions, “How many minutes does it usually take you to fall asleep at bedtime?” and “How many minutes of wake time (waking up in the middle of the night) do you have during a typical night’s sleep?” Self-reported sleep duration was then calculated by subtracting time awake during the night from total available sleep time for each subject.
Questionnaires were collected and entered by blinded research assistants. Adherence data were transmitted automatically to a secure server without involvement of staff members. Statisticians were unblinded during the analysis phase.
Power Analysis
We performed an a priori power analysis based on data from Richards et al12 who observed an improvement in CPAP adherence from 2.5 ± 2.7 h/night to 5.4 ± 2.6 h/night with a behavioral intervention. Assuming a between-patient standard deviation of 2.6, a sample size of 45 subjects per arm yielded 80% power to detect a difference in adherence of 1.5 hours at a significance level of 0.05.
Statistical Approach
All analyses were performed using SAS 9.3 (SAS Institute, Inc). Continuous variables were compared between groups using independent t tests or Wilcoxon rank sum tests; categorical data were compared using Fisher exact tests. Our primary analysis was a comparison of CPAP adherence (hours/night) between arms for 6 months; we conducted a secondary analysis of data collected for 12 months as fewer participants reached this point in the study. To compare adherence between arms we fit mixed-effect models with subject-specific intercepts and slopes over time, using adherence data recorded each night for each participant. Models were adjusted for intervention, follow-up duration, and randomization factors (site, CVD status, sleep study type, and device manufacturer). We added a series of interaction terms (separately) to our fully adjusted model of 6-month adherence data to investigate potential effect modification by baseline questionnaire scores, CVD status, self-reported mask usage during the run-in phase, and recruitment site. Results were considered statistically significant when P < .05 (two-sided). All analyses were intention-to-treat.
Results
Figure 1 shows the flow of participants into the study. Of the 83 randomly assigned participants, 26 in each arm were enrolled for 12-month follow-up, and the remainder were enrolled for 6-month follow-up. Participants were typical for a group with OSA and CVD or risk factors, being predominantly male (67%), 63.9 ± 7.4 years on average, and obese (BMI, 30.6 ± 4.5 kg/m2). The two groups were comparable at baseline in terms of age, sex, anthropometrics, race or ethnicity, and cardiovascular risk factors (Table 1).
Table 1.
Descriptive characteristics in the CPAP-only and CPAP + ME groups at baseline
| Variable | CPAP Only (n = 42) | CPAP + ME (n = 41) |
|---|---|---|
| Descriptive characteristics | ||
| Age at randomization, y | 63.9 ± 7.4 | 63.8 ± 8.3 |
| Ethnicity/race non-Hispanic/white, No. (%) | 36 (85.7) | 36 (87.8) |
| Male sex, No. (%) | 28 (66.7) | 27 (65.9) |
| BMI, kg/m2 | 30.6 ± 4.5 | 31.6 ± 5.9 |
| Neck circumference, cm | 41.2 ± 4.6 | 41.0 ± 3.3 |
| University/college graduate or higher, No. (%) | 24 (57.1) | 25 (61.0) |
| Established cardiovascular disease, No. (%) | 24 (58.5) | 25 (61.0) |
| Office systolic blood pressure, mm Hg | 124.8 ± 19.5 | 127.5 ± 16.2 |
| Office diastolic blood pressure, mm Hg | 71.2 ± 10.6 | 71.3 ± 8.9 |
| Recruited from sleep clinic, No. (%) | 7 (17) | 6 (15) |
| Sleep data | ||
| Home-based sleep study, No. (%) | 34 (81.0) | 35 (85.4) |
| AHI, events/h | 23.7 (15.9, 31.4) | 21.8 (17.4, 31.0) |
| Time Spo2 < 90%, % | 3.7 (0.6, 12.3) | 2.8 (0.5, 10.4) |
| AHI ≥ 30 events/h, No. (%) | 15 (35.7) | 12 (29.3) |
| CPAP level, cm H2O | 9.5 ± 1.7 | 9.6 ± 2.4 |
| Trial duration | ||
| Six months (n = 83), d | 183 ± 24 | 181 ± 16 |
| Twelve months (n = 52), d | 340 ± 49 | 333 ± 34 |
| Questionnaire data | ||
| Epworth Sleepiness Scale score (of 24)a | 7.7 ± 4.2 | 8.4 ± 4.8 |
| SEMSA: Risk perception (of 4)b | 2.4 ± 0.5 | 2.3 ± 0.6 |
| SEMSA: Outcome expectations (of 4)b | 2.8 ± 0.7 | 2.7 ± 0.6 |
| SEMSA: Self efficacy (of 4)b | 2.9 ± 0.7 | 2.8 ± 0.8 |
| PHQ-8 Depression Scale (of 24)c | 3.0 (1.0, 7.0) | 4.0 (2.0, 10.0) |
| WHI Insomnia Rating Scale (of 20)d | 9.3 ± 5.0 | 10.2 ± 4.8 |
Categorical data are presented as No. and %; normally distributed continuous data are presented as mean ± SD; and skewed continuous data are presented as median (lower quartile, upper quartile). AHI = apnea-hypopnea index; ME = motivational enhancement; PHQ-8 = Patient Health Questionnaire 8-item depression scale; SEMSA = Self Efficacy Measure for Sleep Apnea; WHI = Women’s Health Initiative.
Higher score indicates greater sleepiness; ≥ 11 indicates severe daytime sleepiness.
Higher score indicates greater self-efficacy; no commonly used cutoff to indicate high self-efficacy.
Higher score indicates greater depressive symptoms; ≥ 10 indicates major depression.
Higher score indicates greater insomnia symptoms; no commonly used cutoff for symptom severity.
Retention
Two participants in the CPAP-only arm (5%) and three participants in the CPAP + ME arm (7%) refused therapy. These participants were retained in all analyses with adherence as 0.0 h/night. In the CPAP + ME arm, attendance at the first ME session during the baseline appointment was therefore 93%; attendance at the second ME session was 85%. Participation in the ME phone calls at 3, 4, 8, 12, 20, and 32 weeks ranged from 62% to 80%.
Subjects in both arms were offered follow-up appointments with a sleep technologist. Attendance by subjects in the CPAP-only and CPAP + ME arms was 81% and 85%, respectively, at 1 week, 74% and 78% at 1 month, 60% and 71% at 3 months, 45% and 51% at 6 months, and 42% and 62% at 9 months (P > .05 at each time point).
Assessment of Fidelity to Intervention Framework
An independent external rater listened to the audiotape for a random selection of 18 baseline ME sessions (47% of total) and 12 follow-up ME sessions (34% of total). The rater recorded whether each item of a 24-item (baseline) or 37-item (follow-up) checklist had been discussed during each session. Median fidelity was 95% for baseline sessions (range, 71%-100%) and 91% for follow-up sessions (range, 72%-100%).
Effect of Motivational Enhancement on Adherence
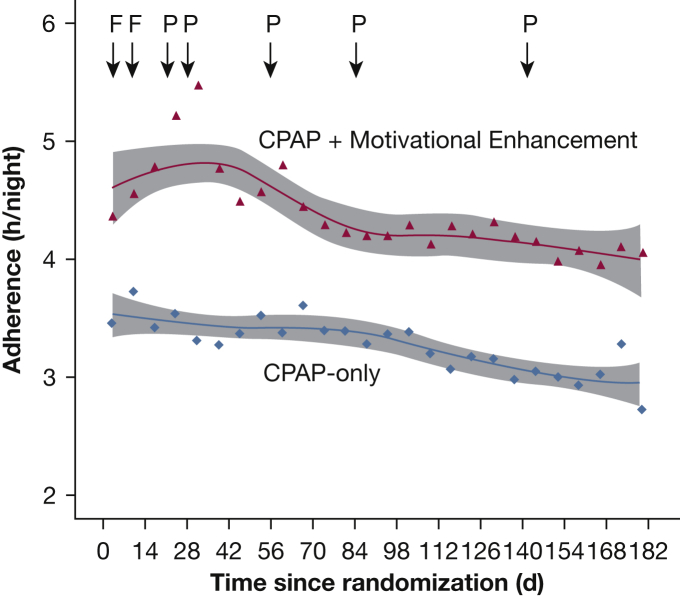
Figure 2 shows adherence in the CPAP-only and CPAP + ME arms for 6 months. For our primary analysis, 83 participants contributed 14,273 nights of data; average adherence across this time was 3.3 ± 2.7 and 4.4 ± 2.9 h/night in the CPAP-only and CPAP + ME arms, respectively. In a mixed model including every night of data and adjusted for intervention and study duration (number of nights), the estimated difference in CPAP adherence between arms was 80 minutes (95% CI, 5.0-155.2; P = .04), favoring the CPAP + ME arm. The estimated difference in adherence between arms was similar in a model further adjusted for randomization factors (99 minutes; 95% CI, 33.0-164.9; P = .003).
Figure 2.
CPAP adherence for 6 months in the CPAP-only and CPAP + motivational enhancement (ME) arms. Each data point represents the mean adherence for each 7-day period. Each line is a fitted LOESS (local regression) curve, with the shaded area representing the 95% CI for the curve. Timing of the face-to-face ME visits are marked as “F”; timing of the phone-based ME visits are marked as “P.”
Our secondary analysis included all available data, that is, 6-month and 12-month data, resulting in 83 participants contributing 22,245 nights of data; average adherence across this time was 3.2 ± 2.8 and 4.3 ± 2.9 h/night in the CPAP-only and CPAP + ME arms, respectively. In our fully adjusted model the difference in adherence between arms was 97 minutes (P = .006), favoring the CPAP + ME group.
Change in CPAP Adherence Over Time
To investigate the change in CPAP adherence over time, we estimated a linear slope for each individual and compared the average slopes between groups. Adherence worsened slightly over time in both groups, but there was no significant difference between groups (P = .56). The median slope in the CPAP-only and CPAP + ME groups were –0.4 min/night (interquartile range, –22.5 to 4.9) and –1.8 min/night (–19.5 to 1.7), respectively.
Impact of ME on Sleep Duration
We investigated whether ME resulted in increased self-reported sleep duration, which may have impacted adherence. There were no significant differences in sleep duration, either over time within arms (P = .95 andP = .87 in the CPAP-only and CPAP + ME groups, respectively) or on average between arms (P = .86).
Effect Modification
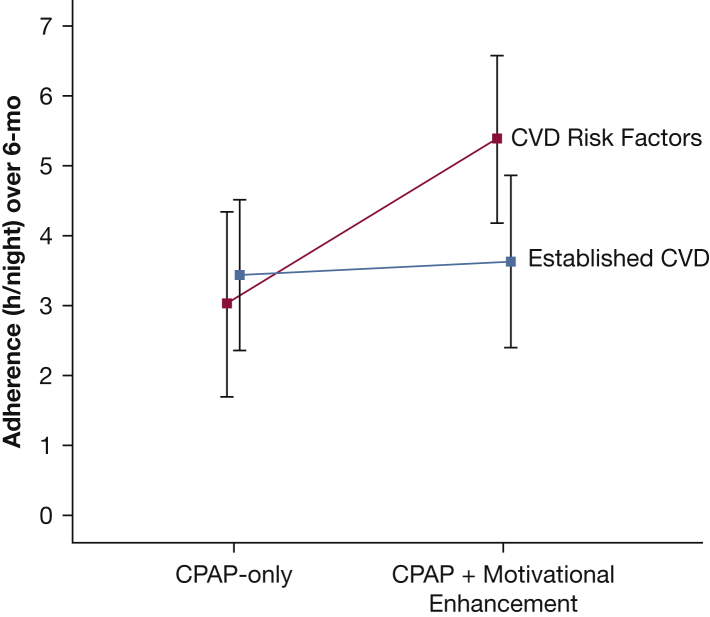
There were no significant interactions with baseline ESS, WHI Insomnia Rating Scale, PHQ-8 Depression Scale, or SEMSA subscale scores, use of the mask during the run-in phase (< 13 or ≥ 13 nights), or recruitment site (sleep clinic vs cardiology or diabetes clinics). ME had a greater impact on adherence among those with CVD risk factors compared with those with established CVD (P = .03 for interaction; Fig 3). Participants with established CVD were significantly younger than those with CVD risk factors (62.1 ± 8.2 years vs 66.2 ± 6.5 years; P = .02), but these groups did not differ on BMI, sex, AHI, or questionnaire scores.
Figure 3.
Significant effect modification by cardiovascular disease status. Mean adherence (with 95% CI) is depicted for those randomly assigned to the CPAP-only and CPAP + motivational enhancement arms, by CVD subgroup (established vs risk factors). The interaction term for CVD status was P = .03. CVD = cardiovascular disease.
Discussion
In this randomized trial of participants with moderate or severe OSA and cardiovascular comorbidity, we have demonstrated increased CPAP adherence of 99 min/night measured for 6 months resulting from an ME intervention compared with CPAP alone. This association remained consistent in a subset of participants followed for 12 months. The ME intervention did not impact adherence by lengthening sleep duration; instead, the intervention focused on behavior change regarding the therapy itself. The difference in adherence of 1.5 h/night (3.3 vs 4.4 h/night on average in the CPAP-only and CPAP + ME arms at 6 months) is highly clinically relevant, particularly when one considers the fact that US-based insurance companies and Medicare require adherence of at least 4.0 h/night to qualify for continuing therapy.23 Similar adherence thresholds are in place internationally; it is therefore important that steps are taken to ensure that this goal is reached. The substantial difference in adherence between groups in our study is noteworthy, given that few participants reported excessive daytime sleepiness at baseline. Further, 80% of participants were recruited from cardiology or diabetes clinics13 and were not actively seeking diagnosis or treatment for OSA.
Of note, the average adherence in our CPAP-only group at 12 months (3.2 h/night) was very similar to what has been observed in the ongoing SAVE trial, which also recruited a sample with OSA and cardiovascular comorbidity and began CPAP without a specific behavioral intervention.7 Subjects who completed our ME intervention demonstrated adherence of 4.3 h/night at 12 months; the range was 0.0 to 8.3 h/night, suggesting wide variability in individual adherence. Prior work by Antic et al3 and Weaver et al2 has demonstrated that different “doses” of CPAP are required to see substantial improvements in subjective and objective daytime sleepiness, as well as functional status. Further research is needed to better understand how to set and manage adherence targets that maximize individual benefits across a range of outcomes.
Our subgroup analyses consistently demonstrated that the participants randomly assigned to ME exhibited greater CPAP adherence compared with control subjects; that is, the intervention was efficacious regardless of baseline self-reported sleepiness, self-efficacy, depression, insomnia symptoms, or recruitment site (sleep clinic vs other clinics). In addition, we found that the ME had a greater impact on adherence in those with CVD risk factors than in those with established CVD. The ME intervention included a component in which participants were encouraged to rate their own health and functional status, to think about how this might differ if they did not have OSA, and to anticipate what changes they might expect if their OSA was treated. It is therefore possible that participants who had recently been informed that they were considered to be at risk of CVD, as opposed to those with established CVD, may have been more susceptible to the increased motivation to engage with CPAP elicited by the intervention to mitigate future possible cardiovascular events. Alternatively, it is possible that patients in the subgroup with established CVD were sicker and therefore had competing demands from other medical treatments, making the ME intervention less efficacious in these participants.
To our knowledge, our study is the first to incorporate night-by-night CPAP adherence into the statistical analysis by adopting a mixed-effect model approach. Prior studies have collapsed adherence into a single data point per participant, whereas our analysis captures the complexity of nightly adherence information that, from prior studies, is known to change with time.24, 25 A further strength of our study is that we have demonstrated increased CPAP adherence specifically in patients with cardiovascular comorbidity and without marked sleepiness. A common assumption among sleep specialists is that patients who do not actively seek a diagnosis of OSA or those who are not symptomatic have little motivation to become established on CPAP. By recruiting largely asymptomatic patients from cardiology and diabetes clinics, we have demonstrated that with ME therapy it is possible to establish good patterns of adherence in patients who have hitherto been considered “difficult to treat.” Our study also had a longer follow-up duration compared with the majority of published randomized trials of CPAP, which have mostly been less than 3 months.
Our study has some limitations. We were not able to follow our full sample of 83 patients for 12 months; however, the differences in adherence at 6 and 12 months were comparable. The participants who were followed to 12 months were those randomly assigned before a certain date, as opposed to those who chose to remain in the study long term; thus, we do not believe that the 12-month data are biased toward those more motivated to use therapy. Our study was by nature open label, which may have introduced some bias; however, adherence was measured objectively, and CPAP initiation and clinical follow-up were delivered uniformly across arms. Another potential source of bias comes from our sample of subjects who were willing to participate in the larger trial that included both CPAP and sham-CPAP arms, and thus may not reflect the general OSA and CVD population. Finally, our sample consisted predominantly of non-Hispanic white subjects, and is therefore not necessarily generalizable.
In conclusion, our study demonstrates that a brief, protocolized ME intervention delivered in addition to standard CPAP appointments resulted in a statistically and clinically significant increase in adherence of 99 min/night for 6 months compared with standard CPAP delivery. This difference in adherence remained consistent in a subset of patients followed for 12 months, suggesting that the beneficial effect of ME was retained after the intervention had been withdrawn. Our trial emphasizes the efficacy of ME for maximizing adherence, and may inform clinical practice given that CPAP adherence remains a problem that limits clinical benefit of therapy.
Acknowledgments
Author contributions: J. P. B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse events. R. W., M. S. A., J. H. W., M. A. M., and S. R. were responsible for study design; C. T., M. G. M., K. J. G., M. R., and C. D. were responsible for study conduct and data collection; J. P. B., R. W., J. W., S. R. P., J. H. W., M. A. M., and S. R. were responsible for analysis and interpretation; and J. P. B., R. W., J. W., M. S. A., C. T., M. G. M., K. J. G., M. R., C. D., S. R. P., J. H. W., M. A. M., and S. R. were responsible for manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. S. A. is a paid employee and stockholder for Philips. S. R. P. has served as a consultant to ApniCure and has received grant funding through the ResMed Foundation. None declared (J. P. B., R. W., J. W., C. T., M. G. M., K. J. G., M. R., C. D., J. H. W., M. A. M., S. R.).
Role of sponsors: The sponsors played no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, and the preparation, review, or approval of the manuscript.
Other contributions: We would like to thank all study participants, as well as research staff including Hannah Buettner, BA, Beatriz Oropeza, MS, Erin Reese, MPH, Tricia Tiu, BS, and Christina Zenobi, BS, and our other study investigators Eldrin Lewis, MD, MPH, and Stuart Quan, MD (both Brigham and Women’s Hospital and Harvard Medical School). We thank Dennis Drotar, PhD, for performing the intervention fidelity assessments.
Footnotes
FUNDING/SUPPORT: This study was supported by NIH NHLBI [Grant 1U34HL105277] and a grant from ResMed Foundation. Equipment was donated by ResMed and Philips Respironics.
References
- 1.Weaver T.E., Grunstein R.R. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver T.E., Maislin G., Dinges D.F. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antic N.A., Catcheside P., Buchan C. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbé F., Durán-Cantolla J., Capote F., Spanish Sleep and Breathing Group Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181(7):718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman M.E., Arnedt J.T., Stanchina M., Millman R.P., Aloia M.S. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest. 2006;130(6):1772–1778. doi: 10.1378/chest.130.6.1772. [DOI] [PubMed] [Google Scholar]
- 6.Bouloukaki I., Giannadaki K., Mermigkis C. Intensive versus standard follow-up to improve continuous positive airway pressure compliance. Eur Respir J. 2014;44(5):1262–1274. doi: 10.1183/09031936.00021314. [DOI] [PubMed] [Google Scholar]
- 7.Chai-Coetzer C.L., Luo Y.M., Antic N.A. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36(12):1929–1937. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aloia M.S., Di Dio L., Ilniczky N., Perlis M.L., Greenblatt D.W., Giles D.E. Improving compliance with nasal CPAP and vigilance in older adults with OAHS. Sleep Breath. 2001;5(1):13–21. doi: 10.1007/s11325-001-0013-9. [DOI] [PubMed] [Google Scholar]
- 9.Lai A.Y., Fong D.Y., Lam J.C., Weaver T.E., Ip M.S. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA: a randomized controlled trial. Chest. 2014;146(3):600–610. doi: 10.1378/chest.13-2228. [DOI] [PubMed] [Google Scholar]
- 10.Aloia M.S., Arnedt J.T., Strand M., Millman R.P., Borrelli B. Motivational enhancement to improve adherence to positive airway pressure in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2013;36(11):1655–1662. doi: 10.5665/sleep.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen S., Smith S.S., Oei T.P., Douglas J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomized controlled trial. J Consult Clin Psychol. 2012;80(1):151–163. doi: 10.1037/a0026302. [DOI] [PubMed] [Google Scholar]
- 12.Richards D., Bartlett D.J., Wong K., Malouff J., Grunstein R.R. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep. 2007;30(5):635–640. doi: 10.1093/sleep/30.5.635. [DOI] [PubMed] [Google Scholar]
- 13.Gleason K., Shin D., Rueschman M. Challenges in recruitment to a randomized controlled study of cardiovascular disease reduction in sleep apnea: an analysis of alternative strategies. Sleep. 2014;37(12):2035–2038. doi: 10.5665/sleep.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaggi HK, Mittleman MA, Bravata DM, et al. Reducing cardiovascular risk through treatment of obstructive sleep apnea: two methodological approaches. Am Heart J. (in-press). [DOI] [PMC free article] [PubMed]
- 15.Iber C., Ancoli-Israel S., Chesson A., Quan S. American Academy of Sleep Medicine; Westchester, IL: 2007. The AASM Manual for the Scoring of Sleep and Associated Events. [Google Scholar]
- 16.Miller W.R., Rollnick S. 2nd ed. Guilford Press; New York, NY: 2002. Motivational Interviewing: Preparing People for Change. [Google Scholar]
- 17.Miller W.R., Rollnick S. 3rd ed. Guilford Press; New York, NY: 2013. Motivational Interviewing: Helping People Change. [Google Scholar]
- 18.Aloia M.S., Smith K., Arnedt J.T. Brief behavioral therapies reduce early positive airway pressure discontinuation rates in sleep apnea syndrome: preliminary findings. Behav Sleep Med. 2007;5(2):89–104. doi: 10.1080/15402000701190549. [DOI] [PubMed] [Google Scholar]
- 19.Johns M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Weaver T.E., Maislin G., Dinges D.F. Self-efficacy in sleep apnea: instrument development and patient perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep. 2003;26(6):727–732. doi: 10.1093/sleep/26.6.727. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K., Spitzer R.L., Williams J.B., Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Levine D.W., Kripke D.F., Kaplan R.M. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 23.Schwab R.J., Badr S.M., Epstein L.J., ATS Subcommittee on CPAP Adherence Tracking Systems An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188(5):613–620. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver T.E., Kribbs N.B., Pack A.I. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 25.Aloia M.S., Goodwin M.S., Velicer W.F. Time series analysis of treatment adherence patterns in individuals with obstructive sleep apnea. Ann Behav Med. 2008;36(1):44–53. doi: 10.1007/s12160-008-9052-9. [DOI] [PubMed] [Google Scholar]