Abstract
Starting from tadalafil as a template, a series of functionalized tetrahydro-b-carboline derivatives have been prepared and identified as novel potent and selective PDE5 inhibitors. Replacing the 3,4-methylenedioxyphenyl at position 6 of tadalafil, together with elongation of the N2-methyl substituent and manipulation of the stereochemical aspects of the two chiral carbons led to the identification of compound XXI, a highly potent PDE5 inhibitor (IC50 = 3 nM). Compound XXI was also highly selective for PDE5 versus PDE3B, PDE4B, and PDE11A, with a selectivity index of 52 and 235 towards PDE5 rather than PDE11 with both cAMP and cGMP as substrate, respectively.
Keywords: beta-Carbolines, PDE5 inhibitors, SAR, Synthesis, Tadalafil
Introduction
Tadalafil is a long-acting phosphodiesterase type-5 (PDE-5) inhibitor that has been widely used to treat male erectile dysfunction (MED) [1, 2]. PDE-5 is highly specific for cyclic guanosine monophosphate (cGMP) hydrolysis and is a key modulator of intracellular cGMP signaling pathways [3, 4]. Recently, PDE-5 inhibitors have been reported to be effective in the treatment of various other non-urologic disorders such as chronic obstructive pulmonary disease, prostate hyperplasia, pulmonary arterial hypertension (PAH), and coronary heart disease [5]. Moreover, the over expression of PDE5 in many tumor cells withdraws the attention to the use of PDE5 inhibitors in targeting cancer, the inhibition of PDE5 results in sustained increase in cGMP levels which in turn modifies mitotic arrest in carcinoma cells with enhanced PDE5 expression [6–8].
Tadalafil cross reactivity with other PDEs, particularly PDE11, is reported. PDE11 is an enzyme of still unknown physiological function but of a controversial role in sperms formation and function [9, 10].
In the present article, we describe the results obtained during our attempt to enlarge the structural diversity of the tadalafil-derived family, this was accomplished through the replacement of the 3,4-methylenedioxyphenyl group at position 6 with p-chlorophenyl substituent, contracting the piperazindione to a hydantoin ring, manipulating the N2-methyl group to ethyl, tert-butyl and p-chlorophenyl, and changing the stereochemical status of the 2 chiral carbons to be R,R, R,S, S, R, and S,S.
Results and discussion
Chemistry
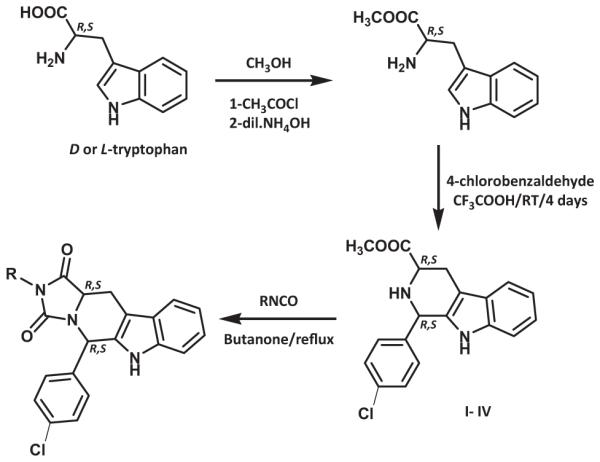
The general synthesis of hydantoin compounds are depicted in Schemes 1 and 2. Pure D- or L-tryptophane methyl ester and p-chlorobenzaldehyde were subjected to a Pictet-Spengler reaction [11]. Since we initially desired access to both the cis- and trans-isomers (I–IV), the reaction was carried under non stereospecific conditions. The cis- and trans-1,3-disubstiuted tetrahydro-β-carbolines (I–IV) were separated by column chromatography, each of the pure diastereomers was allowed to react with commercially available isocyanates to yield the desired cis- and trans-hydantoins.
Scheme 1.
Synthesis of hydantoin tetrahydro-β-carboline derivatives (V–XVI).
Scheme 2.

Synthesis of piperazinedione tetrahydro-β-carboline derivatives (XXI–XXVIII).
Piperazindione derivatives were prepared by the chloroacetylation of pure cis- and trans-1,3-disubstiuted tetrahydro-β-carbolines (I–IV) in the presence of NaHCO3. This step provided the respective chloroethanone derivative in an excellent yield. The piperazinedione diastereomers were then obtained by ring closure of the corresponding chloroacetyl derivatives in the presence of primary amines, namely ethylamine and tert-butylamine.
The assignment of cis-/trans-stereochemistry for the tetrahydro-β-carbolines (I–IV) was based on the detailed study of 13C-NMR spectroscopy data well established in previous literature [12, 13]. Signals for C-1and C-3 in the trans-isomers appear at higher field in the carbon spectrum than the analogous carbons of the corresponding cis-isomer, probably due to the 1,3-interactions present in the trans-tetrahydro-β-carboline isomer. The 1H-NMR signals for the proton at C-1, of the 1,3-disubstituted-tetrahydro-β-carbolines (I–IV) appeared at about δ 5.20–5.70 ppm, upon cyclization to the hydantoin and piperazinedione derivatives the same proton which is now attached to C-5 and C-6, respectively, makes a remarkable downfield shift to δ 5.70–6.93 compared to its respective precursor.
Also, regarding the tetrahydro-β-carbolines a significant difference has been noted between the 2 isomers regarding their melting points and Rf values. A correlation exists between Rf value on TLC and the stereochemistry of the 1,3-disubstituted tetrahydro-β-carbolines. The cis-isomer is generally less polar than the trans-isomer; however, in the hydantoin series, the polarity is reversed, thus, the cis-isomer becomes more polar than the trans-isomer. The difference in both Rf and melting point values are not as significant in the piperazindione series as it is in the hydantoin series.
Interestingly, X-ray quality crystals of the most active compound XXI were grown in ethanol and the crystal structure was obtained using X-ray crystallography. The two hydrogen atoms at C-1 (C-6 in nomenclature) and C-12 (C-12a in nomenclature) are protruding at the same side indicating the proposed R,R absolute configuration and that no epimerization took place, Fig. 1.
Figure 1.

X-ray structure of XXI, showing protrusion of the 2 hydrogens at C-6 (labelled C-1) and C-12a (labelled - C12) in the same direction showing cis-orientation.
Biology
Compounds were evaluated in an in-vitro assay for inhibitory activity against human PDE5; each compound was evaluated in two steps. The first step was the determination of the percentage of inhibition at 50 μM performed in triplicate. For compounds displaying a percentage of inhibition greater than 60%, the IC50 was determined from a concentration-response curve using a range of 8 concentrations (1 nM– 50 μM) with at least two replicates per concentration. The results are shown in Tables 1–4. Moreover, for the most active compound XXI, the selectivity towards other PDEs (PDE3B, PDE4B, and PDE11A) was evaluated, the results are shown in Table 5.
Table 1.
% Inhibition of PDE5 and IC50 values for the tetrahydro-β-carbolines (I–IV)
| Code | Absolute stereochemistry |
% cGMP inhibition (50 μM) |
IC50 (μM) |
|---|---|---|---|
| I | (1R,3R) | 35 | ND* |
| II | (1S,3R) | 4 | ND |
| III | (1S,3S) | 9 | ND |
| IV | (1R,3S) | 48 | ND |
ND = not determined
Table 4.
% Inhibition of PDE5 and IC50 values for the β-carbolines-piperazinedione derivatives (XXI–XXVIII)
| Code | R | Absolute stereochemistry |
% cGMP inhibition (50 μM) |
IC50 (μM) |
|---|---|---|---|---|
| XXI | C2H5 | (6R,12aR) | 102 | 0.003 |
| XXII | C2H5 | (6S,12aR) | 80 | 1.72 |
| XXIII | C2H5 | (6S,12aS) | 45 | ND* |
| XXIV | C2H5 | (6R,12aS) | 87 | 1.2 |
| XXV | C(CH3)3 | (6R,12aR) | 55 | ND |
| XXVI | C(CH3)3 | (6S,12aR) | 10 | ND |
| XXVII | C(CH3)3 | (6S,12aS) | 42 | ND |
| XXVIII | C(CH3)3 | (6R,12aS) | 55 | ND |
ND = not determined
Table 5.
IC50 (μM) of XXI and tadalafil versus an array of PDEs.
| Comp. | PDE3B cAMP | PDE3B cGMP | PDE4B | PDE5A | PDE11A cAMP | PDE11A cGMP | Selectivity index IC50
(PDE11/PDE5) |
|
|---|---|---|---|---|---|---|---|---|
| cAMP/cGMP | cGMP/cGMP | |||||||
| XXI | >50 | >50 | >50 | 0.003 | 0.706 | 0.157 | 235 | 52.3 |
| Tadalafil | >50 | >50 | >50 | 0.007 | 0.295 | 0.050 | 42.1 | 7 |
Based on the introduced structural changes, the following SAR for PDE5 inhibitors can be concluded:
1,3-Disubstiuted THBCs (I–IV) did not show appreciable inhibition to PDE5. As all the active derivatives were of the THBCs-hydantoin or THBCs-piperazinedione skeleton, thus the PDE5 inhibition is determinately linked to the formation of the respective hydantoin, or piperazinedione derivatives. It is worth to mention that all the chloroethanone derivatives (XVII–XX) were also among the least active at the screening dose, this confirms that and integer tetracyclic part is essential for PDE5 inhibition.
Replacing the 3,4-methylenedioxyphenyl group by the p-chlorophenyl group still produces active candidates with appreciable PDE5 inhibitory activity. XXI seems to be the most active compound with IC50 = 3 nM with almost a two-fold higher potency compared with tadalafil. It seems that the size and the position of the chlorine atom allow its fitting in the narrow Q2 pocket of the PDE5 enzyme, Fig. 2. The size of this pocket is very characteristic for PDE5 leading to the belief that it governs the selectivity of the ligands towards the enzyme.
Figure 2.
The overlay of tadalafil (represented by grey ball and stick) and XXI (represented by black ball and stick) in the binding pocket of human PDE5. The active site of PDE5 is divided into three pockets: the metal binding pocket (M), the purine-selective glutamine and hydrophobic clamp pocket (Q) which is further divided into Q1 and Q2 subpockets, and the solvent-filled side pocket (S).
Regarding the nature and size of the substituent on the terminal nitrogen of hydantoin or piperazinedione ring, it has been shown that compounds with ethyl or tert-butyl substituent are more active than their congeners with p-chlorophenyl one e.g. V and IX versus XIII and VIII versus XVI. Thus a small alkyl substituent at position 2 seems favorable for activity.
It is worth to mention that a chlorine substituent at the p-position of the pendant aryl and an ethyl substituent on the nitrogen of the terminal ring are good option for activity and the two changes are in favor of more hydrophobic interaction with the receptor.
The piperazinedione terminal ring derivatives seem to be equipotent to their hydantoin congeners; interestingly, the most potent and selective compound XXI possessed a piperazinedione ring.
Regarding the absolute stereochemical requirement for a PDE5 inhibition, all active compounds of the hydantoin series posses an R absolute configuration at position 5; the most active compound of this series was VIII with (5R,11aS) absolute stereochemistry. The order of activity was as follows: (5R,11aS) > (5R,11aR). None of the hydantoins with C5 with the absolute configuration S showed significant activity. For the piperazindione series, the most active compound XXI was of (6R, 12aR) absolute configuration. Again, except for one compound XXII, all compounds with the 6S absolute configuration did not show appreciable PDE5 inhibition. This indicates that the activity is mainly set by the stereochemistry of the carbon derived from the aldehyde rather than the carbon derived from the amino acid. This opens the horizon towards potent PDE5 inhibitors from L-tryptophan as well.
The most active compound XXI was tested versus an array of other PDEs, namely PDE3B, with both cAMP and cGMP as substrates, PDE4B with cAMP as substrate and PDE11A with both cAMP and cGMP as substrates to decide about its selectivity profile. Interestingly, XXI showed 52 and >200 times selectivity towards PDE5 rather than PDE11 with cGMP and cAMP as substrates, respectively. Meanwhile, tadalafil was only seven and 42-times as selective, respectively. In addition, XXI was almost inactive versus all other tested PDEs. This indicates that XXI might be free from those side effects due to cross reactivity with other PDEs, particularly PDE11.
To further investigate the binding mode of our compounds compared to tadalafil, a docking experiment was applied, whereby the human PDE5 co-crystallized with tadalafil (PDB code 1UDU) was downloaded from the Protein data Bank. The old ligand was removed, tadalafil and XXI were docked to the binding pocket of human PDE5, using MOE 2007.09. The most stable conformers of both compounds were almost overlaid indicating that both of them bind to the binding pocket in a similar fashion, Fig. 2. The 2D-view of the interaction between XXI and the PDE5 binding pocket showed H-bonding with Q817, the protein residue involved in nucleotide recognition; the indole ring showed π-π stacking with the hydrophobic residues lining the cavity of the active sites namely F820 (Fig. 3) and finally the chlorine atom seems to occupy the narrow Q2 pocket occupied by the methylene dioxo group of tadalafil and in lipophilic interaction with M816.
Figure 3.

Detailed mode view showing the docking and interaction of XXI with human PDE5.
On an attempt to explain the difference in potency between XXI (IC50 = 0.003 μM) and its diastereomer XXII (IC50 = 1.72 μM), the latter was docked into the binding pocket of PDE5 using the same docking parameters as the former. Compound XXII differs from XXI only in one stereocenter. The inversion of the 5R center to 5S induces a 180° flip of the central scaffold. The hydrogen bond between Gln817 and the NH-group of the indole moiety together with π-π stacking with F820 present in both tadalafil and all potent novel compounds of R configuration are lost, Fig. 4.
Figure 4.

Detailed mode view showing the docking and interaction of XXII with human PDE5.
Experimental
Chemistry
All starting materials were commercially available and of pure analytical grade. All reactions were carried out under inert gas (nitrogen). Organic extracts were dried over anhydrous Na2SO4. Solvents were removed under reduced pressure using a rotavap. Reaction progress was monitored by TLC, performed on pre-coated silica gel plates (ALUGRAM SIL G/UV254) and detection of the components was made by short UV light. Column chromatography was performed using silica-gel (70–200 μm). Melting points were determined on Buchi Melting Point apparatus and are uncorrected. FTIR spectra were recorded on Nicolet Avatar 380 spectrometer. 1H-spectra were run at 300 MHz and 13C-spectra were run at 75.46 MHz in deuterated chloroform (CDCl3) and dimethylsulfoxide (DMSO). Chemical shifts (δ) were reported in parts per million (ppm) downfield from TMS; multiplicities are abbreviated as: s: singlet, d: doublet, t: triplet, q: quartet, m: multiplet, dd: doublet of doublet, brs: broad. Mass spectra were made on Focus GC/Polaris MS, model 5890, series II, at an ionization potential of 70 eV. Elemental analysis were performed by the Microanalytical Unit, Faculty of Science, Cairo University; the found values were within ±0.4% of the theoretical ones, unless otherwise indicated. For X-ray crystallography, preliminary examination and data collection were performed on Enraf-Nonius CAD-4, Mainz University, Germany.
Methods
General procedures for the preparation of methyl-1-(4-chlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (I–IV)
The appropriate tryptophane methyl ester (10.76 g, 49.3 mmol) and 4-chlorobenzaldehyde (7.62 g, 54.23 mmol) were dissolved in CH2Cl2 (70 mL) and cooled to 0°C in an ice bath. To this solution was added dropwise TFA (8 mL), and the mixture was stirred at room temperature for 4 days under N2 atmosphere. The reaction mixture was then basified with dilute NH4OH solution and extracted with CH2Cl2 (3 × 50 mL). The organic layer was washed with water, brine, dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified and the isomers were separated by column chromatography on silica gel, eluting with CH2Cl2, to give first the appropriate cis-isomer followed by CH2Cl2/CH3OH (99.5:0.5) the trans-isomer.
Methyl (1R,3R)-1-(4 chlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (I)
Yellow powder (2.5 g, 15%); m.p.: 220–223°C; Rf = 0.72 (CH2Cl2/CH3OH, 98:2); 1H-NMR (CDCl3): 7.79 (brs, 1H, NH), 7.53–7.50 (d, 1H, Ar), 7.25–6.81 (m, 7H, Ar), 5.70 (s, 1H, CHPh), 4.02–3.97 (m, 1H, CHCOOCH3), 3.87 (s, 3H, OCH3), 3.37–3.17 (m, 1H, CHaHb), 3.05–2.99 (m, 1H, CHaHb); MS (EI): m/z 342 (M+ + 2), m/z 340 (M+; 100%); IR (cm−1): 3374, 1726; anal. calcd. (C19H17ClN2O2): C, 66.96; H, 5.03; N, 8.22. Found: C, 66.76; H, 5.09; N, 8.54.
Methyl (1S,3R)-1-(4-chlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (II)
Buff powder (4.19 g, 25%); m.p.:189-191°C; Rf = 0.48 (CH2Cl2/CH3OH, 98:2); 1H-NMR (CDCl3): 7.63 (brs, 1H, NH), 7.58–7.57 (d, 1H, Ar), 7.18–7.17 (d, 2H, Ar), 7.15–7.14 (d, 2H, Ar), 6.98–6.92 (m, 4H, Ar), 5.45 (s, 1H, CHPh), 4.03–3.90 (dd, 1H, CHCOOCH3), 3.74 (s, 3H, OCH3), 3.34–3.24 (dd, 1H, CHaHb),3.19–3.16 (d, 1H, CHaHb); MS (EI): m/z 342 (M+ + 2), m/z 340 (M+; 100%); IR (cm−1): 3335, 1708; anal. calcd. (C19H17ClN2O2): C, 66.96; H, 5.03; N, 8.22. Found: C, 66.78; H, 5.10; N, 8.44.
Methyl (1S,3S)-1-(4-chlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (III)
Yellow powder (7.49 g, 45%); m.p.: 221-224°C; Rf = 0.73 (CH2Cl2/CH3OH, 98:2); 1H-NMR (CDCl3): 8.89 (brs, 1H, NH), 8.25–8.22 (d, 1H, Ar), 7.94–7.91 (d, 1H, Ar), 7.76–7.73 (d, 2H, Ar), 7.56–7.52 (d, 2H, Ar), 7.41–7.13 (m, 2H, Ar), 5.24(s, 1H, CHPh), 4.00–3.95 (dd, 1H, CHCOOCH3), 3.83 (s, 3H, OCH3), 3.27–3.21 (dd, 1H, CHaHb), 3.06–2.96 (m, 1H, CHaHb), 2.02 (brs, 1H, NH); MS (EI): m/z 342 (M+ + 2), m/z 340 (M+; 100%); IR (cm−1): 3382, 1726; anal. calcd. (C19H17ClN2O2): C, 66.96; H, 5.03; N, 8.22. Found: C, 66.99; H, 5.12; N, 8.60.
Methyl (1R,3S)-1-(4-chlorophenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (IV)
Buff powder (1.83 g, 11%); m.p.: 190-193°C; Rf = 0.49 (CH2Cl2/CH3OH, 98:2); 1H-NMR (CDCl3): 7.81(brs, 1H, NH), 7.60–7.58 (d, 2H, Ar), 7.55–7.54 (d, 2H, Ar), 7.33–7.13 (m, 4H, Ar), 5.46 (s, 1H, CHPh), 3.90 (dd, 1H, CHCOOCH3), 3.73 (s, 3H, OCH3), 3.36–3.25 (dd, 1H, CHaHb), 3.21–3.11 (dd, 1H, CHaHb); MS (EI): m/z 342 (M+ + 2), m/z 340 (M+ 100%); IR (cm−1): 3332, 1708; anal. calcd. (C19H17ClN2O2): C, 66.96; H, 5.03; N, 8.22. Found: C, 66.70; H, 5.20; N, 8.50.
General procedures for the preparation of 2-alkyl or tert-butyl or aryl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo [1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (V-XX)
The appropriate isocyanate (1.6 mmol) was added to a well stirred solution of the appropriate β-carboline I-IV (0.34 g, 1 mmol) in methyl ethyl ketone (10 mL) and the mixture was stirred at reflux for 16 h under nitrogen atmosphere. The product was purified using by column chromatography on silica gel, eluting with CH2Cl2.
(5R,11aR)-2-Ethyl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (V)
Yellow powder (0.11 g, 30%); m.p. 230–232°C; Rf = 0.42 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 8.42 (brs, 1H, NH), 7.52–7.49 (d, 2H, Ar), 7.46–7.43 (d, 2H, Ar), 7.23–7.09 (m, 4H, Ar), 5.72 (s, 1H, CHPh), 4.33–4.27 (dd, 1H, CHC(O)N), 3.49–3.41 (m, 1H, CHaCHb), 3.03–2.98 (q, 2H, NCH2), 2.87–2.85 (m, 1H, CHaHb), 1.18–1.09 (t, 3H, CH3); 13C-NMR: 171.1, 136.9, 136.6, 135.2, 132.6, 128.9, 128.8, 125.9, 122.7, 120.0, 118.3, 113.9, 107.1, 57.7 (C5), 55.8 (C11a), 33.4, 22.2, 13.17; MS (EI): m/z 381 (M+ + 2), m/z 379 (M+; 100%); IR (cm−1): 3318, 1760, 1702, anal. calcd. (C21H18ClN3O): C, 66.40; H, 4.78; N, 11.06. Found: C, 66.49; H, 4.79; N, 11.20.
(5S,11aR)-2-Ethyl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (VI)
White powder (0.05 g,15%); m.p.: 222–225°C; Rf = 0.73 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 8.22 (brs, 1H, NH), 7.93–7.91 (d, 2H, Ar), 7.66–7.64 (d, 2H, Ar), 7.63–7.61 (m, 2H, Ar), 7.59–7.55 (dd, 1H, Ar), 7.54–7.50 (dd, 1H, Ar), 6.63 (s, 1H, CHPh), 4.64–4.58 (dd, 1H, CHC(O)N), 3.98–3.89 (q, 2H, NCH2), 3.87–3.81, (dd, 1H, CHaCHb), 3.29–3.18 (m, 1H, CHaHb), 1.60–1.54 (t, 3H, CH3); 13C-NMR: 167.5, 149.9, 132.7, 131.8, 130.1, 124.9, 124.7, 124.5, 121.2, 118.2, 115.4, 113.6, 106.4, 103.6, 48.3 (C5), 46.4 (C11a), 28.9, 24.8, 18.4; IR (cm−1): 3418, 1764, 1690; MS (EI): m/z 381 (M+ + 2), m/z 379 (M+; 100%); anal. calcd. (C21H18ClN3O): C, 66.40; H, 4.78; N, 11.06. Found: C, 66.39; H, 4.70; N, 11.28.
(5S,11aS)-2-Ethyl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (VII)
White powder (0.19 g, 50%); m.p.: 232–235°C; Rf = 0.43 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 7.61 (brs, 1H, NH), 7.58–7.57 (d, 2H, Ar), 7.33–7.32 (d, 2H, Ar), 7.24–7.15 (m, 4H, Ar), 5.8 (s, 1H, CHPh), 4.41–4.36 (dd, 1H, CHC(O)N), 3.63–3.57 (q, 2H, NCH2), 3.56–3.50 (d, 1H, CHaCHb), 3.10–3.01 (dd, 1H, CHaHb), 1.24–1.19 (t, 3H, CH3); 13C-NMR: 167.6, 149.7, 137.1, 132.7, 129.2, 129.1, 124.7, 124.4, 123.1, 121.8, 120.3, 118.5, 111.2, 105.8, 103.3, 107.4, 57.9 (C5), 56.1 (C11a), 33.6, 22.4, 13.4; MS (EI): m/z 381 (M+ + 2), m/z 379 (M+; 100%), IR (cm−1): 3244, 1730, 1692; anal. calcd. (C21H18ClN3O): C, 66.40; H, 4.78; N, 11.06. Found: C, 66.59; H, 4.91; N, 11.23.
(5R,11aS)-2-Ethyl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (VIII)
White powder (0.28 g, 75%); m.p.: 220–222°C; Rf = 0.72 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 8.26 (brs, 1H, NH), 7.93–7.90 (d, 2H, Ar), 7.73–7.70 (d, 2H, Ar), 7.69–7.50 (m, 4H, Ar), 6.62 (s, 1H, CHPh), 4.64–4.57 (dd, 1H, CHC(O)N), 3.98–3.89 (q, 2H, NCH2), 3.87–3.81 (d, 1H, CHaCHb), 3.29–3.18 (dd, 1H, CHaHb), 1.59–1.54 (t, 3H, CH3); 13C-NMR: 172.5, 154.9, 137.7, 136.8, 135.0, 129.9, 129.7, 129.4, 128.7, 127.1, 126.2, 123.2, 120.4, 118.6, 111.4, 108.5, 53.2 (C5), 51.4 (C11a), 33.9, 23.4, 13.6; IR (cm−1): 3305, 1764, 1703; MS (EI): m/z 381 (M+ + 2), m/z 379 (M+; 100%); anal. calcd. (C21H18ClN3O): C, 66.40; H, 4.78; N, 11.06. Found: C, 66.49; H, 4.97; N, 11.30.
(5R,11aR)-2-tert-Butyl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (IX)
Buff crystals (0.31 g, 75%); m.p.: 288–290°C; Rf = 0.72 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 8.50 (brs, 1H, NH), 7.87–7.83 (d, 2H, Ar), 7.78–7.75 (d, 2H, Ar), 7.33–7.14 (m, 4H, Ar), 5.73 (s, 1H, CHPh), 4.27–4.22 (dd, 1H, CHC(O)N), 3.49–3.43 (dd, 1H, CHaCHb), 3.06–2.96 (m, 1H, CHaHb), 1.59 (s, 9H, CH3); IR (cm−1): 3323, 1755, 1692; MS (EI): m/z 409 (M+ + 2), m/z 407 (Mþ; 100%); anal. calcd. (C23H22N3O4): C, 67.73; H, 5.44; N, 10.30. Found: C, 67.93; H, 5.61; N, 10.01.
(5S,11aR)-2-tert-Butyl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (X)
White powder (0.2 g, 50%); m.p.: 196–199°C; Rf = 0.89 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 7.81 (s, 1H, NH), 7.60–7.56 (d, 2H, Ar), 7.33–7.29 (d, 2H, Ar), 7.29–7.16 (m, 4H, Ar), 6.24 (s, 1H, CHPh), 4.18–4.12 (dd, 1H, CHC(O)N), 3.50–3.43 (dd, 1H, CHaCHb), 2.91–2.81 (m, 1H, CHaHb), 1.63 (s, 9H, CH3); IR (cm−1): 3325, 1757, 1692; MS (EI): m/z 409 (M+ + 2), m/z 407 (M+; 100%); anal. calcd. (C23H22ClN3O): C, 67.73; H, 5.44; N, 10.30. Found: C, 67.52; H, 5.31; N, 10.32.
(5S,11aS)-2-tert-Butyl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (XI)
White powder (0.12 g, 30%); m.p.: 285–287°C; Rf = 0.71 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 8.43 (brs, 1H, NH), 7.86–7.83 (d, 2H, Ar), 7.78–7.75 (d, 2H, Ar), 7.59–7.57 (d, 1H, Ar), 7.48–6.14 (m, 3H, Ar), 5.73 (s, 1H, CHPh), 4.26–4.21 (dd, 1H, CHC(O)N), 3.49–3.43 (dd, 1H, CHaCHb), 3.05–2.96 (m, 1H, CHaHb), 1.59 (s, 9H, CH3). IR (cm−1): 3402, 1764, 1692; MS (EI): m/z 409 (M+ + 2), m/z 407 (M+; 100%); anal. calcd. (C23H22ClN3O2): C, 67.73; H, 5.44; N, 10.30. Found: C, 67.64; H, 5.47; N, 10.05.
(5R,11aS)-2-tert-Butyl-5-(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (XII)
White crystals (0.31 g, 75%); m.p.: 195–198°C; Rf = 0.89 (CH2Cl2/Ar), 7.32–7.30 (d, 2H, Ar), 7.24–7.16 (m, 4H, Ar), 6.25 (s, 1H, CHPh), 4.18–4.13 (dd, 1H, CHC(O)N), 3.51–3.44 (dd, 1H, CHaCHb), 2.91–2.81 (m, 1H, CHaHb), 1.63 (t, 9H, CH3); IR (cm−1): 3405, 1765, 1698; MS (EI): m/z 409 (M+ + 2), m/z 407 (M+; 100%); anal. calcd. (C23H22ClN3O2): C, 67.73; H, 5.44; N, 10.30. Found: C, 67.80; H, 5.69; N, 10.03.
(5R,11aR)-2,5-Bis(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (XIII)
Buff powder (0.32 g, 72%); m.p.: 270–273°C; Rf = 0.78 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 7.64 (brs, 1H, NH), 7.61–7.57 (d, 2H, Ar), 7.40–7.39 (d, 2H, Ar), 7.31–7.30 (d, 2H, Ar), 7.26–7.27 (d, 2H, Ar), 7.25–7.19 (m, 4H, Ar), 5.89 (s, 1H, CHPh), 4.61–4.55 (dd, 1H, CHC(O)N), 3.66–3.60 (dd, 1H, CHaCHb), 3.22–3.16 (m, 1H, CHaHb); IR (cm−1): 3330, 1770, 1722; MS (EI): m/z 466 (M+ + 4), m/z 464 (M+ + 2), m/z 462 (M+; 100%); anal. calcd. (C25H17Cl2N3O2): C, 64.95; H, 3.71; N, 9.09. Found: C, 64.15; H, 3.91; N, 8.94.
(5S,11aR)-2,5-Bis(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (XIV)
White powder (0.29 g, 63%); m.p.: 152–155°C; Rf = 0.92 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 7.85 (brs, 1H, NH), 7.62–7.60 (d, 2H, Ar), 7.19 (m, 10H, Ar), 6.38 (s, 1H, CHPh), 4.48–4.43 (dd, 1H, CHC(O)N), 3.64–3.57 (dd, 1H, CHaCHb), 3.10–3.01 (m, 1H, CHaHb); IR (cm−1): 3336, 1772, 1720. MS (EI): m/z 466 (M+ + 4), m/z 464, (M+ + 2), m/z 462 (M+; 100%); anal. calcd. (C25H17Cl2N3O2): C, 64.95; H, 3.71; N, 9.09. Found: C, 64.85; H, 3.53; N, 9.02.
(5S,11aS)-2,5-Bis(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (XV)
White powder (0.39 g, 85%); m.p.: 269–272°C; Rf = 0.78 (CH2Cl2/ MeOH, 99:1); 1H-NMR (CDCl3): 7.63 (brs, 1H, NH), 7.61–7.58 (d, 2H, Ar), 7.32–7.30 (d, 2H, Ar), 7.27–7.17 (m, 8H, Ar), 5.89 (s, 1H, CHPh), 4.60–4.55 (dd, 1H, CHC(O)N), 3.66–3.58 (dd, 1H, CHaCHb), 3.24–3.16 (m, 1H, CHaHb); IR (cm−1: 3320, 1773, 1710; MS (EI): m/z 466 (M+ + 4), m/z 464 (M+ + 2), m/z 462 (M+; 100%); anal. calcd. (C25H17Cl2N3O2): C, 64.95; H, 3.71; N, 9.09. Found: C, 64.60; H, 3.87; N, 8.90.
(5R,11aS)-2,5-Bis(4-chlorophenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1’,5’:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (XVI)
White powder (0.12 g, 25%); m.p.: 150–153°C; Rf = 0.91 (CH2Cl2/MeOH, 99:1); 1H-NMR (CDCl3): 7.82 (brs, 1H, NH), 7.62–7.60 (d, 2H, Ar), 7.43–7.42 (d, 2H, Ar), 7.36–7.35 (d, 2H, Ar), 7.46–7.19 (m, 6H, Ar), 6.39 (s, 1H, CHPh), 4.49–4.44 (dd, 1H, CHC(O)N), 3.65–3.58 (dd, 1H, CHaCHb), 3.12–3.02 (m, 1H, CHaHb); IR (cm−1): 3337, 1772, 1710; MS (EI): m/z 466 (M+ + 4), m/z 464 (M+ + 2, 64%), m/z 462 (M+; 100%); anal. calcd. (C25H17Cl2N3O2): C, 64.95; H, 3.71; N, 9.09. Found: C, 65.00; H, 4.01; N, 9.05.
General procedures for the preparation of methyl-1-(4-chlorophenyl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate
To a well stirred solution of of the appropriate β-carboline I–IV (1.93 g, 5.7 mmol) and NaHCO3 (0.57 g, 6.89 mmol) in CHCl3 (40 mL) was added (1.1 mL, 13.69 mmol) chloroacetylchloride dropwise at 0°C. The mixture was then stirred under N2 for 1 h. The mixture was then diluted with CH2Cl2 washed with a solution of NaHCO3, dried over anhydrous Na2SO4. The residue was then crystallized from diethyl ether.
Methyl (1R,3R)-1-(4-chlorophenyl)-2-(chloroacetyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (XVII)
Yellow powder (2.25 g, 95%); m.p.: 225–228°C; Rf = 0.76 (CH2Cl2/MeOH, 98:2); 1H-NMR (CDCl3): 10.86 (brs, 1H, NH), 7.56–7.54 (d, 2H, Ar),7.38–7.36 (d, 2H, Ar), 7.31–7.28 (d, 1H, Ar), 7.13–7.01 (m, 3H, Ar), 6.83 (s, 1H, CHPh), 5.23–5.21 (d, 1H, CHCOOCH3), 4.86–4.81 (d, 1H, COCHaHbCl), 4.47–4.42 (d, 1H, COCHaHbCl), 3.33 (s, 3H, OCH3), 3.14–3.06 (dd, 1H, CHaCHb), 2.96–2.50 (d, 1H, CHaHb); IR (cm−1): 3247, 1728, 1658; MS (EI): m/z 421 (M+ + 4), m/z 419 (M+ + 2), m/z 417 (M+; 100%); anal. calcd. (C21H18Cl2N2O3): C, 60.44; H, 4.35; N, 6.71. Found: C, 60.11; H, 4.26; N, 6.81.
Methyl (1S,3R)-1-(4-chlorophenyl)-2-(chloroacetyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (XVIII)
Green powder (2.31 g, 97%); m.p.: 110–112°C; Rf = 0.66 (CH2Cl2/MeOH, 98:2); 1H-NMR (CDCl3): 7.72 (brs, 1H, NH), 7.55–7.53 (d, 2H, Ar), 7.29–7.27 (d, 2H, Ar), 7.24–7.11 (m, 4H, Ar), 6.10 (s, 1H, CHPh), 5.23–5.20 (d, 1H, CHCOOCH3), 4.13–4.09 (d, 1H, COCHaHbCl), 4.04–4.00 (d, 1H, COCHaHbCl), 3.65 (s, 3H, OCH3), 3.14–3.06 (dd, 1H, CHaCHb), 2.90–2.56 (d, 1H, CHaHb); IR (cm−1): 3319, 1737, 1658; MS (EI): m/z 421 (M+ + 4), m/z 419 (M+ + 2), m/z 417 (M+; 100%); anal. calcd. (C21H18Cl2N2O3): C, 60.44; H, 4.35; N, 6.71. Found: C, 60.72; H, 4.45; N, 6.85.
Methyl (1S, 3S)-1-(4- chlorophenyl)-2-(chloroacetyl)- 2, 3, 4, 9-tetrahydro-1H-b-carboline-3-carboxylate (XIX)
Yellow (2.25, 95%); m.p.: 228–232°C; Rf = 0.76 (CH2Cl2/MeOH, 98:2); 1H-NMR (CDCl3): 10.86 (brs, 1H, NH), 7.57–7.55 (d, 2H, Ar), 7.39–7.36 (d, 2H, Ar), 7.31–7.29 (d, 1H, Ar), 7.13–7.00 (m, 3H, Ar), 6.82 (s, 1H, CHPh), 5.24–5.22 (d, 1H, CHCOOCH3), 4.45–4.41 (d, 1H, COCHaHbCl), 4.38–4.34 (d, 1H, COCHaHbCl), 3.29 (s, 3H, OCH3), 3.13–3.06 (dd, 1H, CHaCHb), 2.96–2.49 (d, 1H, CHaHb); IR (cm−1): 3246, 1730, 1658; MS (EI): m/z 421 (M+ + 4), m/z 419 (M+ + 2), m/z 417 (M+; 100%); anal. calcd. (C21H18Cl2N2O3): C, 60.44; H, 4.35; N, 6.71. Found: C, 60.46; H, 4.47; N, 6.88.
Methyl (1R,3S)-1-(4-chlorophenyl)-2-(chloroacetyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (XX)
Yellowish green powder (2.19 g, 92%); m.p.: 107–110°C; Rf = 0.67 (CH2Cl2/MeOH, 98:2); 1H-NMR (CDCl3): 10.86 (brs, 1H, NH), 7.57–7.55 (d, 2H, Ar), 7.13–7.00 (m, 6H, Ar), 6.92 (s, 1H, CHPh), 5.20–5.16 (d, 1H, CHCOOCH3), 4.48–4.44 (d, 1H, COCHaHbCl), 3.98–3.94 (d, 1H, COCHaHbCl), 3.29 (s, 3H, OCH3), 3.13–3.06 (dd, 1H, CHaCHb), 2.98–2.47 (d, 1H, CHaHb); IR (cm−1): 3326, 1727, 1650, MS (EI): m/z 421 (M+ + 4;), m/z 419 (M+ + 2), m/z 417 (M+; 100%), anal. calcd. (C21H18Cl2N2O3): C, 60.44; H, 4.35; N, 6.71. Found: C, 60.79; H, 4.65; N, 6.92.
General procedures for the preparation of 2-ethyl or tert-butyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione
A solution of the of the appropriate chloroacetyl derivative XVII–XX (0.58 g, 1.4 mmol) and the appropriate amine (2.8 mmol) in methanol (25 mL) was heated to reflux under a nitrogen atmosphere for 16 h. The reaction mixture was cooled to room temperature and evaporated to dryness under reduced pressure. The residue was dissolved in CH2Cl2, and the organic layer was washed with water, dried over Na2SO4, filtered, and concentrated to dryness. The crude product was then purified using column chromatography eluting with CH2Cl2/CH3OH (99:1). The product was crystallized from ethanol.
(6R,12aR)-2-Ethyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (XXI)
Buff crystals (0.22 g, 40%); m.p.: 300–303βC; Rf = 0.5 (CH2Cl2/MeOH, 97:3); 1H-NMR (CDCl3): 8.03 (brs, 1H, NH), 7.64–7.62 (d, 2H, Ar), 7.29–7.30 (d, 2H, Ar), 7.24–7.22 (d, 1H, Ar), 7.21–7.19 (d, 1H, Ar), 7.18–7.19 (d, 1H, Ar), 7.61–7.60 (d, 1H, Ar), 6.21 (s, 1H, CHPh), 4.33–4.28 (dd, 1H, CHC(O)N), 4.13–4.07 (d, 1H, CHaHbC(O)N), 3.95–3.89 (d, 1H, CHaHbC(O)N), 3.83–3.76 (dd, 1H, CHaHb), 3.73–3.66 (q, 2H, NCH2), 3.27–3.17 (m, 1H, CHaHb), 1.23–1.18 (t, 3H, CH3); 13C-NMR: 67.1, 165.7, 139.8, 136.6, 132.6, 133.5, 132.1, 128.8, 128.5, 125.1, 126.1, 122.6, 120.2, 118.6, 111.2, 106.9, 56.2 (C6), 49.6 (C12a), 41.2, 29.5, 23.7, 12.1; IR (cm−1): 3208, 1657, 1650; MS (EI): m/z 396 (M+ + 2), m/z 394 (M+; 100%); anal. calcd. (C22H20ClN3O2): C, 67.09; H, 5.12; N, 10.67. Found: C, 67.29; H, 5.24; N, 10.50.
(6S,12aR)-2-Ethyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (XXII)
Buff powder (0.14 g, 25%); m.p.: 255–258°C; Rf = 0.53 (CH2Cl2/MeOH, 97:3); 1H-NMR (CDCl3): 7.98 (brs, 1H, NH), 7.64–7.62 (d, 2H, Ar), 7.47–7.45 (d, 2H, Ar), 7.25–7.23 (d, 1H, Ar), 7.19–7.15 (m, 3H, Ar), 6.22 (s, 1H, CHPh), 4.33–4.28 (dd, 1H, CHC(O)N), 4.13–4.07 (d, 1H, CHaHbC(O)N), 3.94–3.86 (d, 1H, CHaHbC(O)N), 3.82–3.75 (dd, 1H, CHaHb), 3.42–3.35 (q, 2H, NCH2), 3.27–3.17 (m, 1H, CHaHb), 1H, CHaHb), 3.42–3.35 (q, 2H, NCH2), 3.27–3.17 (m, 1H, CHaHb), 1.23–1.18 (t, 3H, CH3); 13C-NMR: 167.2, 165.8, 139.8, 136.6, 133.6, 132.2, 128.8, 128.6, 126.1, 125.9, 122.7, 120.2, 118.6, 111.3, 106.9, 55.3 (C6), 48.4 (C12a), 41.2, 29.5, 23.7, 12.1; IR (cm−1: 3279, 1658, 1652; MS (EI): m/z 396 (M+ + 2), m/z 394 (M+; 100%); anal. calcd. (C22H20ClN3O2): C, 67.09; H, 5.12; N, 10.67. Found: C, 66.99; H, 5.01; N, 10.47.
(6S,12aS)-2-Ethyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (XXIII)
Golden yellow powder (0.16 g, 30%); m.p.: 300–303°C; Rf = 0.5 (CH2Cl2/MeOH, 97:3); 1H-NMR (CDCl3): 7.91 (brs, 1H, NH), 7.64–7.61 (d, 2H, Ar), 7.30–7.29 (d, 2H, Ar), 7.25–7.15 (m, 4H, Ar), 6.22 (s, 1H, CHPh), 4.34–4.29 (dd, 1H, CHC(O)N), 4.14–4.08 (d, 1H, CHaHbC(O)N), 3.95–3.89 (d, 1H, CHaHbC(O)N), 3.83–3.76 (dd, 1H, CHaHb), 3.73–3.66 (q, 2H, NCH2), 3.27–3.18 (m, 1H, CHaHb), 1.23–1.18 (t, 3H, CH3); IR (cm−1): 3281, 1658, 1643; MS (EI): m/z 396 (M+ + 2), m/z 394 (M+; 100%); anal. calcd. (C22H20ClN3O2): C, 67.09; H, 5.12; N, 10.67. Found: C, 67.09; H, 5.00; N, 10.47.
(6R,12aS)-2-Ethyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (XXIV)
Yellow powder (0.14 g, 25%); m.p.: 257–259°C; Rf = 0.51 (CH2Cl2/MeOH, 97:3); 1H-NMR (CDCl3): 7.85 (brs, 1H, NH), 7.64–7.62 (d, 2H, Ar), 7.79–7.72 (d, 2H, Ar), 7.59–7.40(dd, 1H, Ar), 7.25–7.24 (d, 1H, Ar), 7.21–7.17 (m, 2H, Ar), 6.21 (s, 1H, CHPh), 4.35–4.29 (dd, 1H, CHC(O)N), 4.14–4.08 (d, 1H, CHaHbC(O)N), 3.95–3.90 (d, 1H, CHaHbC(O)N), 3.83–3.76 (dd, 1H, CHaHb), 3.73–3.66 (q, 2H, NCH2), 3.27–3.18 (m, 1H, CHaHb), 1.23–1.18 (t, 3H, CH3); IR (cm−1): 3190, 1657, 1631; MS (EI): m/z 396 (M+ + 2), m/z 394 (M+; 100%); anal. calcd. (C22H20ClN3O2): C, 67.09; H, 5.12; N, 10.67. Found: C, 67.19; H, 4.99; N, 10.64.
(6R,12aR)-2-tert-Butyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (XXV)
White powder (0.05 g, 10%); m.p.: 230–232°C; Rf = 0.13 (CH2Cl2/MeOH, 97:3); 1H-NMR (CDCl3): 7.88 (brs, 1H, NH), 7.60–7.56 (d, 2H, Ar), 7.32–7.28 (d, 2H, Ar), 7.20–7.14 (m, 4H, Ar), 6.93 (s, 1H, CHPh), 4.92–4.97 (d, 1H, CHC(O)N), 4.73–4.71(d, 1H, CHaHbC(O)N), 3.66–3.60 (d, 1H, CHaHbC(O)N), 3.44–3.36 (m, 1H, CHaHb), 3.09–2.99 (m, 1H, CHaHb), 1.19 (s, 9H, CH3); IR (cm−1): 3266, 1648, 1657; MS (EI): m/z 424 (M+ + 2), m/z 422 (M+; 100%); anal. calcd. (C24H24ClN3O2): C, 68.32; H, 5.73; N, 9.96. Found: C, 68.40; H, 5.49; N, 10.11.
(6S,12aR)-2-tert-Butyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (XXVI)
Buff powder (0.09 g, 15%); m.p.: 133–136°C; Rf = 0.13 (CH2Cl2/MeOH, 97:3); 1H-NMR (CDCl3): 9.2 (brs, 1H, NH), 7.97–7.94 (d, 2H, Ar), 7.52–7.49 (d, 2H, Ar), 7.32–7.29 (d, 1H, Ar), 7.19–7.09 (m, 3H, Ar), 6.46 (s, 1H, CHPh), 4.94–4.92 (d, 1H, CHC(O)N), 4.63–4.61 (dd, 1H, CHaHbC(O)N), 4.12–4.07 (d, 1H, CHaHbC(O)N), 3.83–3.63 (m, 1H, CHaHb), 3.14–2.15 (m, 1H, CHaHb), 1.27 (s, 9H, CH3); IR (cm−1): 3300, 1642, 1658; MS (EI): m/z 424 (M+ + 2), m/z 422 (M+; 100%); anal. calcd. (C24H24ClN3O2): C, 68.32; H, 5.73; N, 9.96. Found: C, 68.29; H, 5.50; N, 10.09.
(6S,12aS)-2-tert-Butyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (XXVII)
Yellow powder (0.09 g, 15%); m.p.: 228–230°C; Rf = 0.14 (CH2Cl2/MeOH, 97:3); 1H-NMR (CDCl3): 8.99 (brs, 1H, NH), 8.25–8.23 (d, 2H, Ar), 7.66–7.64 (d, 2H, Ar), 7.62–7.60 (d, 1H, Ar), 7.23–7.09 (m, 3H, Ar), 6.73 (s, 1H, CHPh), 4.84–4.82 (d, 1H, CHC(O)N), 4.25–4.19 (d, 1H, CHaHbC(O)N), 3.89–3.83 (d, 1H, CHaHbC(O)N), 3.68–3.58 (m, 1H, CHaHb), 3.09–2.99 (m, 1H, CHaHb), 1.27 (s, 9H, CH3); IR (cm−1): 3298, 1684, 1649; MS (EI): m/z 424 (M+ + 2), m/z 422 (M+; 100%); anal. calcd. (C24H24ClN3O2): C, 68.32; H, 5.73; N, 9.96. Found: C, 68.14; H, 5.79; N, 10.12.
(6R,12aS)-2-tert-Butyl-6-(4-chlorophenyl)-2,3,6,7,12,12a-hexahydropyrazino[1’,2’:1,6]pyrido[3,4-b]indole-1,4-dione (XXVIII)
Yellowish green powder (0.07 g, 12%); m.p.: 137–140°C; Rf = 0.13 (CH2Cl2/MeOH, 97:3); 1H-NMR: 9.54 (brs, 1H, NH), 8.09–8.05 (d, 2H, Ar), 7.79–7.75 (d, 2H, Ar), 7.55–7.53 (d, 1H, Ar), 7.34–7.14 (m, 3H, Ar), 6.81 (s, 1H, CHPh), 4.92–4.86 (dd, 1H, CHC(O)N), 4.12–4.02 (d, 1H, CHaHbC(O)N), 3.82–3.76 (d, 1H, CHaHbC(O)N), 3.65–3.59 (m, 1H, CHaHb), 3.09–3.08 (m, 1H, CHaHb), 1.27 (s, 9H, CH3); IR (cm−1): 3279, 1680, 1649; MS (EI): m/z 424 (M+ + 2), m/z 422 (M+; 100%); anal. calcd. (C24H24ClN3O2): C, 68.32; H, 5.73; N, 9.96. Found: C, 68.29; H, 5.70; N, 10.07.
Biological evaluation
All the synthesized compounds were evaluated for their inhibitory properties versus recombinant PDE5. For all assays, tadalafil (PDE5/PDE11 inhibitor) was used for comparison. Compound XXI was further tested against PDE3A cAMP, PDE3B cGMP, PDE4B, PDE11A cAMP, PDE11A cGMP to prove its selectivity.
Phosphodiesterase inhibitory activity
PDE activity was measured using an adaptation of the IMAP1 fluorescence polarization phosphodiesterase assay (Molecular Devices, Sunnyvale, CA, USA). PDE hydrolysis of the fluorescent-labeled substrate allows it to bind the IMAP1 reagent, which increases fluorescence polarization (FP). The assay used fluorescein (Fl)-cAMP and tetramethylrhodamine (TAMRA)-cGMP as substrates. The different excitation and emission spectra of the substrates (485–530 nm for Fl and 530–590 nm for TAMRA) allowed simultaneous measurement of cAMP and cGMP hydrolysis in the same well. The assays were performed in 96-well microtiter plates using a reaction buffer containing 10 mM Tris-HCl (pH 7.2), 10 mM MgCl2, 0.05% NaN3, and 0.1% phosphate-free bovine serum albumin as the carrier. Each well contained 20 μL of recombinant enzyme (BPS Biosciences, San Diego, CA, USA) and 10 μL inhibitor. The reaction was initiated by the addition of 10 μL of a substrate solution containing 50 nM Fl-cAMP and/or TAMRA-cGMP. After incubating at room temperature for 60 min the reaction was terminated by adding 120 μL of binding solution. FP was measured with a BioTek Synergy 4 (BioTek Instruments, Winooski, VM, USA) [14].
Table 2.
% Inhibition of PDE5 and IC50 values for the β-carbolines-hydantoin derivatives (V–XVI)
| Code | R | Absolute stereochemistry |
% cGMP inhibition (50 μM) |
IC50 (μM) |
|---|---|---|---|---|
| V | C2H5 | (5R,11aR) | 85 | 1.1 |
| VI | C2H5 | (5S,11aR) | 47 | ND* |
| VII | C2H5 | (5S,11aS) | 30 | ND |
| VIII | C2H5 | (5R,11aS) | 102 | 0.33 |
| IX | C(CH3)3 | (5R,11aR) | 100 | 0.6 |
| X | C(CH3)3 | (5S,11aR) | 54 | ND |
| XI | C(CH3)3 | (5S,11aS) | 20 | ND |
| XII | C(CH3)3 | (5R,11aS) | 83 | >10μM |
| XIII | p-chlorophenyl | (5R,11aR) | 22 | ND |
| XIV | p-chlorophenyl | (5S,11aR) | 29 | ND |
| XV | p-chlorophenyl | (5S,11aS) | 33 | ND |
| XVI | p-chlorophenyl | (5R,11aS) | 84 | 1.09 |
ND = not determined
Table 3.
% Inhibition of PDE5 and IC50 values for the chloroacetyl tetrahydro-β-carbolines derivatives (XVII–XX)
| Code | Absolute stereochemistry |
% cGMP inhibition (50 μM) |
IC50 (μM) |
|---|---|---|---|
| XVII | (1R,3R) | 25 | ND* |
| XVIII | (1S,3R) | 6 | ND |
| XIX | (1S,3S) | 6 | ND |
| XX | (1R,3S) | 78 | 2.7 |
ND = not determined
Acknowledgments
This study was supported in part by The German University in Cairo research fund. The authors are grateful to the Alexander Von Humboldt for donating some of the instruments used.
Footnotes
The authors have declared no conflicts of interest.
References
- [1].Daugan A, Grondin P, Ruault C, Le Monnier de Gouville AC, Coste H, Kirilovsky J, Hyafil F, Labaudiniere R. J. Med. Chem. 2003;46:4525–4532. doi: 10.1021/jm030056e. [DOI] [PubMed] [Google Scholar]
- [2].Daugan A, Grondin P, Ruault C, Le Monnier de Gouville AC, Coste H, Linget JM, Kirilovsky J, Hyafil F, Labaudiniere R. J. Med. Chem. 2003;46:4533–4542. doi: 10.1021/jm0300577. [DOI] [PubMed] [Google Scholar]
- [3].Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Circ. Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- [4].Zhang L, Zhang Z, Zhang RL, Cui Y, LaPointe MC, Silver B, Chopp M. Brain. Res. 2006;1118:192–198. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- [5].van Driel MF. Ned. Tijdschr. Geneeskd. 2006;150:1613–1616. [PubMed] [Google Scholar]
- [6].Zhu B, Vemavarapu L, Thompson WJ, Strada SJ. J. Cell. Biochem. 2005;94:336–350. doi: 10.1002/jcb.20286. [DOI] [PubMed] [Google Scholar]
- [7].Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. J. Exp. Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Piazza GA, Thompson WJ, Pamukcu R, Alila H, Clark M, Liu L, Fetter J, Gresh W, Klein-Szanto J, Farnell D, Eto I, Grubbs C. Cancer Res. 2001;61:3961–3968. [PubMed] [Google Scholar]
- [9].Francis SH. Int. J. Impot. Res. 2005;17:467–468. doi: 10.1038/sj.ijir.3901377. [DOI] [PubMed] [Google Scholar]
- [10].Weeks JL, Zoraghi R, Beasley A, Sekhar KR, Francis SH, Corbin JD. Int. J. Impot. Res. 2005;17:5–9. doi: 10.1038/sj.ijir.3901283. [DOI] [PubMed] [Google Scholar]
- [11].Whaley WM, Govindachari TR. Org. React. 1951;6:151–190. [Google Scholar]
- [12].Ungemach F, Soerens D, Weber R, DiPierro M, Campos O, Mokry P, Cook JM, Silverston JV. J. Am. Chem. Soc. 1980;102:6976–6984. [Google Scholar]
- [13].Sandrin J, Soerens D, Cook JM. Heterocycles. 1976;4:1249–1255. [Google Scholar]
- [14].Huang W, Zhang Y, Sportsman JR. J. Biomol. Screen. 2002;7:215–219. doi: 10.1177/108705710200700305. [DOI] [PubMed] [Google Scholar]



