Abstract
Objectives:
Dual antiplatelet therapy (DAPT), consisting of clopidogrel and aspirin, is the main-stay treatment of acute coronary syndromes (ACS). However, major adverse cardiovascular events may occur even in patients undergoing DAPT, and this has been related to the variable pharmacodynamic efficacy of these drugs, especially clopidogrel. Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are novel inflammatory markers for cardiovascular risk stratification, which may reflect an inflammatory state and thus high on-treatment platelet reactivity (HPR).
Methods:
We investigated the usefulness of PLR and NLR in predicting HPR in clopidogrel-treated patients with ACS. A total of 244 patients were enrolled in this study, and 43 of them were nonresponsive to clopidogrel.
Results:
Logistic regression analysis indicated that PLR was significantly associated with HPR (P < 0.001). Using a cutoff level of 331, PLR predicted HPR with a sensitivity of 73% and a specificity of 69% (odds ratio: 376.15, 95% confidence interval = 37.813–3741.728 P < 0.001, receiver operating characteristic curve: 0.885).
Conclusions:
We suggest that more attention should be paid to the PLR values of these patients on admission to identify individuals who may not benefit from clopidogrel during the course of ACS.
Keywords: Acute coronary syndrome, clopidogrel, drug resistance, lymphocyte, neutrophil, platelet
Clopidogrel is a platelet P2Y12 receptor blocker that is frequently used at present. It is used with aspirin in patients undergoing percutaneous coronary interventions (PCIs) or in patients who have an acute coronary syndrome (ACS) to decrease the risk of subsequent major adverse cardiovascular events (MACE), such as stent thrombosis or recurrence of ACS. However, MACE may occur despite the use of the recommended dual antiplatelet therapy, and this clinical condition has been mainly related to the variable pharmacodynamic efficacy of these drugs, especially clopidogrel.[1] In addition to this circumstance, patients in a pro-inflammatory state, such as patients with diabetes mellitus, have also demonstrated high on-treatment platelet reactivity (HPR) independent from CYP2C19 gene polymorphism. These patients have heightened platelet activation[2,3] and a higher percentage of circulating immature platelets. Theoretically, these features may counteract the inhibitory effect of clopidogrel on platelets.[4,5]
During sustained inflammation, lymphocyte counts decrease as a result of increased lymphocyte apoptosis. The resulting inflammatory conditions lead to increased proliferation in megakaryocytic series and relative thrombocytosis. A previous study revealed an association between high circulating platelet count and MACE in patients with severe coronary artery disease (CAD).[6] Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are new prognostic markers that integrate the risk prediction of these two parameters into one. PLR, in particular, offers information about both the aggregation and inflammation pathways and it may be more valuable than either platelet or lymphocyte count alone in the prediction of inflammatory burden, and thus HPR. Herein, we investigated the usefulness of novel cardiovascular risk markers (PLR and NLR) in predicting HPR in clopidogrel-treated patients with ACS.
Methods
Study Population and Study Protocol
This study is a single-center and retrospectively designed study. The study protocol was approved by a local noninvasive Ethics Committee. We screened 262 patients who were hospitalized due to ACS between January 2011 and December 2014 in our clinic. Among the 262 patients screened, we enrolled 244 patients whose personal and hospital data were sufficient. Eighty-one patients were diagnosed with non ST-elevation myocardial infarction (NSTEMI), and 163 patients were diagnosed with STEMI. A loading dose of 300 mg was administered to each patient on admission, and this was followed by a daily dose of 75 mg. In the case of primary PCI, the patients were administered a 600 mg loading dose of clopidogrel just before the procedure.
Demographic and clinical variables of the patients (including age, gender, and history of diabetes mellitus, hyperlipidemia, hypertension, and smoking) were recorded. A detailed physical examination was performed for all of the patients included in the study, and they were questioned about their history of previous MI, diabetes mellitus, smoking, hypertension, noncardiac diseases, and family history of CAD. Arterial hypertension was defined as having at least three repeated measurements of blood pressure above 140 mmHg systolic and 90 mmHg diastolic or active use of antihypertensive drugs. Diabetes mellitus was considered in patients with fasting plasma glucose levels above 126 mg/dL in at least two different measurements or current use of anti-diabetic drugs. Smoking was defined as active smoking at the time of the cardiovascular event date. Ex-smokers were not counted as active smokers and were classified with patients who had never smoked. A positive family history for CAD was defined as having a history of CAD or sudden cardiac death in a first-degree relative before the age of 55 years for men and 65 years for women. STEMI was defined as acute chest pain and persistent ≥2 mm ST-segment elevation (>20 min) in at least in two contiguous leads. NSTEMI was defined as acute chest pain with transient ST-segment depression or T-wave inversion, flat T waves, pseudo-normalization of T waves, or no electrocardiogram changes at presentation accompanied by the detection of a rise and/or fall of cardiac biomarker values (preferably cardiac troponin) with at least one value above the 99th percentile upper reference limit.
Patients with advanced valvular heart disease clinically decompensated congestive heart failure, malignancy, hematological disorders, severe renal or hepatic insufficiency, systemic inflammatory conditions, active infection, autoimmune disorders, and patients using steroids were excluded from the study.
Blood samples were collected at rest in a fasting state after careful antecubital vein puncture with a 21-gauge needle. Collection of blood samples was performed in the morning, usually between 7:00 a.m. and 10:00 a.m. The blood samples for HPR were collected 72 h after the first dose, and the last dose intake of aspirin or clopidogrel was administered 24 ± 1 h before the collection of blood samples to guarantee assessment of the exact level of platelet inhibition. The first few milliliters of blood were disposed of to avoid spontaneous platelet activation.
Assessment of High On-treatment Platelet Reactivity
HPR was evaluated using adenosine diphosphate (ADP)-induced platelet aggregometry, which was conducted using a multiplate electrode aggregometry (MEA) device called the Multiplate Analyzer (Dynabyte, Munich, Germany). The multiplate analyzer analyses the function of platelets in whole blood at 37°C via attachment of platelets on metal electrodes, resulting in a change of the electrical impedance, which was simultaneously recorded. Hirudin-anticoagulated whole blood was then diluted and stirred for 3 min in the test cuvettes. Finally, ADP in a concentration of 6.4 mmol/L (ADP test) was added, and aggregation was continually recorded for 5 min. The increase of impedance caused by the attachment of platelets to metal electrodes was detected and transformed to arbitrary aggregation units, which were plotted against time. Aggregation measured with MEA was reported as the area under the curve of arbitrary units (AU-min), and HPR was detected as a change in maximal aggregation ≤20% from the baseline.
Platelet-to-lymphocyte Ratio and Neutrophil-to-lymphocyte Ratio Analysis
Samples for PLR analysis were drawn on admission and analyzed within 1 h after sampling by a Beckman Coulter LH 780 Analyzer (Pasadena, California, USA). The blood samples were stored in ethylenediaminetetraacetic acid-containing tubes. PLR was defined as the absolute platelet count divided by the absolute lymphocyte count. NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count.
Statistical Analysis
SPSS Version 15.0 (SPSS Inc., Chicago, Illinois, USA) was used for the statistical analysis. Continuous variables are described as means and standard deviation. Categorical variables are presented as percentages. The normality of distribution for continuous variables was confirmed with the Kolmogorov–Smirnov test. An independent-sample t-test or the Mann–Whitney U-test was used for continuous variables according to the distribution pattern of the continuous variables. A Chi-square test was used for categorical variables. A receiver operating characteristic (ROC) curve analysis was performed to determine the optimum cutoff PLR value to predict the existence of HPR. Independent associations between HPR and independent variables were evaluated by logistic regression analysis by including all parameters showing a P < 0.1 on multivariate analysis (platelet count, lymphocyte count, neutrophil count, NLR, and PLR) [Table 1]. A two-tailed P < 0.05 was considered to indicate a statistically significant difference between the groups.
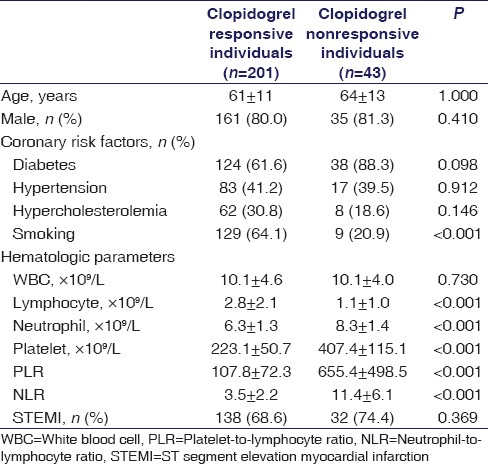
Table 1.
Baseline demographic characteristics of the study population
Results
A total of 244 patients with ACS (72% men) were enrolled in this study. Forty-three members of the study population were nonresponsive to clopidogrel (mean age: 64 ± 13), and 201 were responsive (mean age: 61 ± 11). Of these 244 patients, 18 (7.3%) underwent primary PCI. The great majority of the patients ([92.7%], n = 226) received a 300 mg loading dose, whereas the rest received a loading dose of 600 mg. The mean age of the study population was 62 ± 12. The baseline demographic, biochemical, and hematological characteristics of the groups can be seen in Table 1. Multivariate logistic regression analysis indicated that PLR was significantly associated with HPR (P < 0.001) [Table 2]. The cut-off value of PLR was calculated with an ROC curve and using a cut-off level of 331; PLR predicted HPR with a sensitivity of 73% and a specificity of 69% (odds ratio: 376.15, 95% confidence interval = 37.813–3741.728 P < 0.001, area under ROC curve: 0.885) [Figure 1].
Table 2.
Logistic regression analysis to determine independent variables significantly associated with clopidogrel resistance
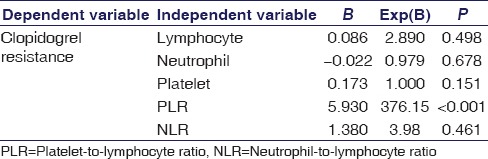
Figure 1.
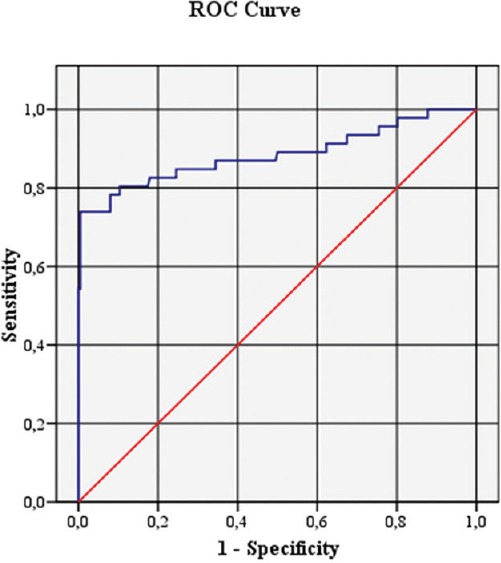
The receiver operating characteristic curve analysis of platelet-to-lymphocyte ratio for predicting high on-treatment platelet reactivity (odds ratio: 376.15, 95% confidence interval = 37.813–3741.728 P < 0.001, area under receiver operating characteristic curve: 0.885)
Discussion
Previous studies have outlined the interindividual variability in the platelet inhibitory effects of clopidogrel.[7] Several underlying mechanisms have been suggested for clopidogrel nonresponsiveness, including noncompliance, variations in pharmacokinetics, intestinal absorption, CYP3A4 metabolization, and systemic inflammation. Inflammation plays an important role in HPR. Inflammation at the site of an atherosclerotic plaque is the major determining factor in the progression and clinical outcome of cardiovascular diseases, including stroke, peripheral vascular disease, and CAD. Therefore, attention has been paid to several markers of systemic inflammation related to HPR, including CRP, von Willebrand factor, and fibrinogen.[8] The presence and severity of increased endothelial dysfunction and platelet reactivity are potentially related to the interindividual variability of clopidogrel efficacy.[9] Ge et al. demonstrated a significant link between inflammatory markers and platelet function resulting in HPR.[10] According to Caruso et al., the upregulation of circulating and platelet adhesion molecules and the presence of a defective platelet antioxidant system suggest that oxidative stress and inflammatory signals might favor platelet hyperactivity, which may be associated with clopidogrel response variability.[11]
PLR is a novel hematological parameter that indicates the inflammatory and prothrombotic state of the patient. High PLR values have been associated with poor prognosis in patients with cardiovascular diseases; however, there are limited data about the association between PLR and HPR.[12,13,14,15] In the current study, we demonstrated that high PLR level was significantly associated with HPR in clopidogrel-treated patients with ACS. This study showed that a PLR value over 331 on admission predicted HPR with a sensitivity of 73%. To the best of our knowledge, this study is the first report investigating the relationship between PLR and HPR.
The initiation and expansion of atherosclerosis in coronary arteries are influenced by many contributing factors. Inflammation plays a central role in all phases of atherosclerosis, which eventually results in thrombotic consequences.[16,17] Lymphocytopenia is a usual finding during the course of chronic inflammatory states due to increased lymphocyte apoptosis. In addition, leukocyte production in bone marrow tends to shift toward increasing neutrophils and decreasing lymphocytes as a response to a chronic inflammatory state. Lymphocytes exhibit a more convenient immunological response; however, neutrophils demonstrate a much more destructive inflammatory reaction.[18] The diagnostic and prognostic practicability of a low lymphocyte count have been previously indicated in patients with ACS, and Ommen et al. found low lymphocyte count to be significantly associated with survival in patients with stable CAD.[19] They suggested that a low lymphocyte count was a novel prognostic indicator in patients with stable CAD. Therefore, it is reasonable to suppose that the lymphocyte count represents an early marker of systemic inflammation.
Leukocyte count is a cheap and easily accessible indicator of the inflammatory response. It has been suggested that neutrophils participate in each step leading to acute coronary events and can expose pro-oxidant and pro-thrombotic mediators, causing endothelial damage and thrombocyte aggregation. Previous studies have revealed that an increase in total white blood cell (WBC) is related with MACE after acute MI.[20] After the realization of a more essential role for immune response in the pathogenesis of CAD, attention has been directed to leukocyte subgroups, especially the NLR. As a combined inflammatory marker, NLR defines the effects of a nonspecific inflammatory response mediated by neutrophils as well as the subsequent regulatory immune response involving lymphocytes. Çiçek et al. demonstrated that the combination of PLR and NLR can be useful for the prediction of in-hospital and long-term mortality in patients undergoing primary percutaneous primary intervention.[21] In this study, we demonstrated that NLR level alone was not associated with HPR in clopidogrel-treated patients with ACS.
Previously conducted studies have shown that 600 mg dose of clopidogrel was associated with higher levels of platelet inhibition and lower mean posttreatment reactivity of ADP than the 300 mg loading dose.[22] In the CURRENT OASIS-7 (Double-dose versus standard-dose clopidogrel and high dose versus low-dose aspirin in individuals undergoing PCI for ACS) trial, a total of 25,087 patients with ACS were randomized to a high-dose regimen (600 mg loading dose of clopidogrel, followed by 150 mg/day for 1 week) or the standard regimen (300 mg on the 1st day followed by 75 mg/day). A total of 600 mg loading dose of clopidogrel in ACS + PCI population demonstrated some benefits. This trial, however, indicated a significant reduction in definite stent thrombosis.[23] In the CLEAR PLATELETS (Clopidogrel Loading with Eptifibatide to Arrest the Reactivity of Platelets) study, 600 mg loading dose was associated with a superior pharmacodynamic antiplatelet activity compared to a 300 mg loading dose of clopidogrel.[24]
In 2015, Sharma et al. conducted a study to evaluate the specific relationship between circulating blood leukocytes, troponin I, and cardiovascular risk factors. They demonstrated that the neutrophils, lymphocytes, and total WBC along with its ratios predicted mortality and are more likely to be elevated in the presence of cardiovascular risk factors.[25] In our study, we did not investigate other hematological parameters except total WBC, lymphocyte, neutrophil counts, PLR, and NLR regarding association with HPR. Among these parameters, multivariate logistic regression analysis indicated that only PLR was significantly associated with HPR (P < 0.001).
There are also some confounding factors in our study population such as smoking, diabetes mellitus and hypertension related to the existence of HPR. Enhanced clopidogrel response in smokers, defined as the smokers’ paradox, is not universal but was observed only in cytochrome P450 CYP1A2 A-allele carriers which indicate a genotype-dependent impact of smoking on clopidogrel responsiveness.[26] In our study, the existence of smokers’ paradox was also confirmed. Hypertension and diabetes were also considered to be other risk factors for HPR in recently conducted studies; however, in our study, we did not observe such kind of relationships.[27,28]
In our study, the PLR values of participants were significantly associated with AU-min values, which support the hypothesis that PLR as an indicator of inflammatory state in the body may be an independent parameter for predicting HPR in patients with ACS. Increased proliferation in megakaryocytic series and relative thrombocytosis are consequences of the continuing inflammatory state in the body, and they cause a prothrombotic condition. It has been stated previously that healthy individuals with increased platelet counts have an augmented risk of experiencing cardiovascular events. High platelet and low lymphocyte counts have been demonstrated to be risk factors for worse cardiovascular outcomes in the previous studies.[29] High PLR, as a novel prognostic marker, combines the risk prediction potential of these two parameters into one. The advantage of PLR calculation could be that it provides a picture of the extent of both aggregation and inflammation and it could be more reliable than either lymphocyte or platelet count alone in the prediction of coronary inflammatory burden and thus in the prediction of HPR.
Conclusions
In this study, we found that PLR value at the time of admission was significantly associated with the existence of HPR in patients hospitalized due to ACS. We believe that more attention should be paid to the PLR values of these patients on admission to identify individuals who may not benefit from clopidogrel therapy during the course of ACS.
Study Limitations
This study has a few limitations. The first is the retrospective design of the study. The present study was not an interventional study; therefore, the results of the study should not be interpreted as causative but rather associative and hypothesis-generating. Another limitation is the variations in the presence of clinical predictors of clopidogrel response, such as genotyping variants. Additional validation cohorts are required to confirm the applicability of these results to other patients suffering from similar clinical conditions.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Gurbel PA, Tantry US. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents?. platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents. Circulation. 2012;125:1276–87. doi: 10.1161/CIRCULATIONAHA.111.031195. [DOI] [PubMed] [Google Scholar]
- 2.Gachet C, Aleil B. Testing antiplatelet therapy. Eur Heart J. 2008;10(Suppl A):A28. [Google Scholar]
- 3.Bouman HJ, van Werkum JW, Hackeng CM, Verheugt FW, Ten Berg JM. The importance of anticoagulant agents in measuring platelet aggregation in patients treated with clopidogrel and aspirin. J Thromb Haemost. 2008;6:1040–2. doi: 10.1111/j.1538-7836.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 4.Michos ED, Ardehali R, Blumenthal RS, Lange RA, Ardehali H. Aspirin and clopidogrel resistance. Mayo Clin Proc. 2006;81:518–26. doi: 10.4065/81.4.518. [DOI] [PubMed] [Google Scholar]
- 5.Buch AN, Singh S, Roy P, Javaid A, Smith KA, George CE, et al. Measuring aspirin resistance, clopidogrel responsiveness, and postprocedural markers of myonecrosis in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2007;99:1518–22. doi: 10.1016/j.amjcard.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, et al. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99:1055–61. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 7.Iijima R, Ndrepepa G, Mehilli J, Bruskina O, Schulz S, Schömig A, et al. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost. 2007;98:852–7. [PubMed] [Google Scholar]
- 8.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84:613–7. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- 9.Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008;12:1422–8. doi: 10.1007/s11605-008-0554-3. [DOI] [PubMed] [Google Scholar]
- 10.Ge H, Zhou Y, Liu X, Yang Q, Nie X, Wang Z, Guo Y, et al. Relationship Between Plasma Inflammatory Markers and Platelet Aggregation in Patients With Clopidogrel Resistance After Angioplasty. Angiology. 2012;63:62–6. doi: 10.1177/0003319711406432. [DOI] [PubMed] [Google Scholar]
- 11.Caruso R, Rocchiccioli S, Gori AM, Cecchettini A, Giusti B, Parodi G, et al. Inflammatory and antioxidant pattern unbalance in “clopidogrel resistant” patients during acute coronary syndrome. Mediators Inflamm 2015. 2015:710123. doi: 10.1155/2015/710123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yüksel M, Yildiz A, Oylumlu M, Akyüz A, Aydin M, Kaya H, et al. The association between platelet/lymphocyte ratio and coronary artery disease severity. Anatol J Cardiol. 2015;15:640–7. doi: 10.5152/akd.2014.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozcan Cetin EH, Cetin MS, Aras D, Topaloglu S, Temizhan A, Kisacik HL, et al. Platelet to lymphocyte ratio as a prognostic marker of in-hospital and long-term major adverse cardiovascular events in ST-segment elevation myocardial infarction. Angiology. 2016;67:336–45. doi: 10.1177/0003319715591751. [DOI] [PubMed] [Google Scholar]
- 14.Akboga MK, Canpolat U, Yayla C, Ozcan F, Ozeke O, Topaloglu S, et al. Association of platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology. 2016;67:89–95. doi: 10.1177/0003319715583186. [DOI] [PubMed] [Google Scholar]
- 15.Akboga MK, Canpolat U, Balci KG, Akyel A, Sen F, Yayla C, et al. Increased platelet to lymphocyte ratio is related to slow coronary flow. Angiology. 2016;67:21–6. doi: 10.1177/0003319715574625. [DOI] [PubMed] [Google Scholar]
- 16.Sönmez O, Ertas G, Bacaksiz A, Tasal A, Erdogan E, Asoglu E, et al. Relation of neutrophil-to-lymphocyte ratio with the presence and complexity of coronary artery disease: An observational study. Anadolu Kardiyol Derg. 2013;13:662–7. doi: 10.5152/akd.2013.188. [DOI] [PubMed] [Google Scholar]
- 17.Kaya H, Ertas F, Islamoglu Y, Kaya Z, Atilgan ZA, Çil H, et al. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost. 2014;20:50–4. doi: 10.1177/1076029612452116. [DOI] [PubMed] [Google Scholar]
- 18.Zouridakis EG, Garcia-Moll X, Kaski JC. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am J Cardiol. 2000;86:449–51. doi: 10.1016/s0002-9149(00)00963-2. [DOI] [PubMed] [Google Scholar]
- 19.Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79:812–4. doi: 10.1016/s0002-9149(96)00878-8. [DOI] [PubMed] [Google Scholar]
- 20.Husser O, Bodi V, Sanchis J, Nunez J, Mainar L, Chorro FJ, et al. White blood cell subtypes after STEMI: Temporal evolution, association with cardiovascular magnetic resonance – Derived infarct size and impact on outcome. Inflammation. 2011;34:73–84. doi: 10.1007/s10753-010-9209-0. [DOI] [PubMed] [Google Scholar]
- 21.Çiçek G, Açikgoz SK, Bozbay M, Altay S, Ugur M, Uluganyan M, et al. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio combination can predict prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2015;66:441–7. doi: 10.1177/0003319714535970. [DOI] [PubMed] [Google Scholar]
- 22.Ray S. Clopidogrel resistance: The way forward. Indian Heart J. 2014;66:530–4. doi: 10.1016/j.ihj.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta SR, Tanguay JF, Eikelboom JW. CURRENT-OASIS 7 trial investigators Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): A randomised factorial trial. Lancet. 2010;376:1233–43. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 24.Gurbel PA, Bliden KP, Zaman KA, Yoho JA, Hayes KM, Tantry US. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: Results of the clopidogrel loading with eptifibatide to arrest the reactivity of platelets (CLEAR PLATELETS) study. Circulation. 2005;111:1153–9. doi: 10.1161/01.CIR.0000157138.02645.11. [DOI] [PubMed] [Google Scholar]
- 25.Sharma KH, Shah KH, Patel I, Patel AK, Chaudhari S. Do circulating blood cell types correlate with modifiable risk factors and outcomes in patients with acute coronary syndrome (ACS)? Indian Heart J. 2015;67:444–51. doi: 10.1016/j.ihj.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edem E, Kirdök AH, Kinay AO, Tekin ÜI, Tas S, Alpaslan E, et al. Does “smoker's paradox” exist in clopidogrel-treated Turkish patients with acute coronary syndrome. Platelets. 2016;27:240–4. doi: 10.3109/09537104.2015.1083544. [DOI] [PubMed] [Google Scholar]
- 27.Akturk IF, Caglar FN, Erturk M, Tuncer N, Yalcin AA, Surgit O, et al. Hypertension as a risk factor for aspirin and clopidogrel resistance in patients with stable coronary artery disease. Clin Appl Thromb Hemost. 2014;20:749–54. doi: 10.1177/1076029613481102. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa I, Park HS, Yokoyama S, Wada T, Hironaka Y, Motoyama Y, et al. Influence of diabetes mellitus and cigarette smoking on variability of the clopidogrel-induced antiplatelet effect and efficacy of active management of the target P2Y12 reaction unit range in patients undergoing neurointerventional procedures. J Stroke Cerebrovasc Dis. 2016;25:163–71. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Thomson SP, Gibbons RJ, Smars PA, Suman VJ, Pierre RV, Santrach PJ, et al. Incremental value of the leukocyte differential and the rapid creatine kinase-MB isoenzyme for the early diagnosis of myocardial infarction. Ann Intern Med. 1995;122:335–41. doi: 10.7326/0003-4819-122-5-199503010-00003. [DOI] [PubMed] [Google Scholar]


