Figure 4.
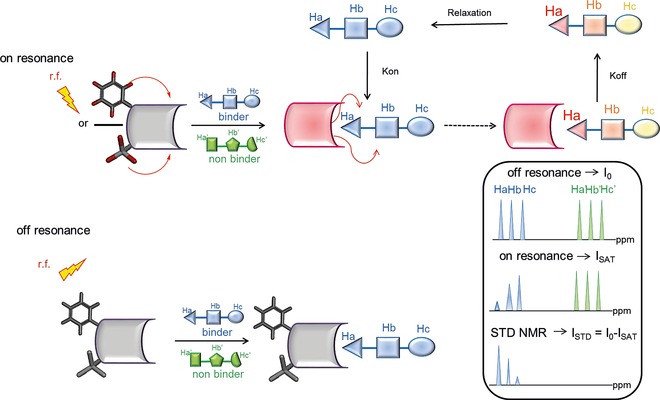
Schematic representation of the STD NMR method. During on‐resonance (upper panel), frequency‐selective irradiation (r.f.), applied for a sustained period (saturation time), causes saturation of the receptor protein. Then, the saturation is transferred by spin diffusion through intermolecular NOEs to the ligand–protein interface, that is, to the bound compounds during their residence time in the protein binding site. Next, by chemical exchange, the ligand molecules go into solution, where the saturated state persists, owing to their small R 1 (enthalpic relaxation) values, and can be detected. In the off‐resonance experiment (lower panel), r.f. saturation is applied in an off‐resonance region from both receptor and ligand, producing a reference spectrum. As a rule of thumb, an irradiation time of 2 s and a 100‐fold excess of ligand give good results. Given the high ligand/protein ratios, only a relatively small amount of protein is required for the measurements (10−9–10−6 m). It is worth noting that receptor resonances are not usually visible, as the protein concentration in solution is very low, and they easily can be deleted by R 2 relaxation filtering prior to detection.
