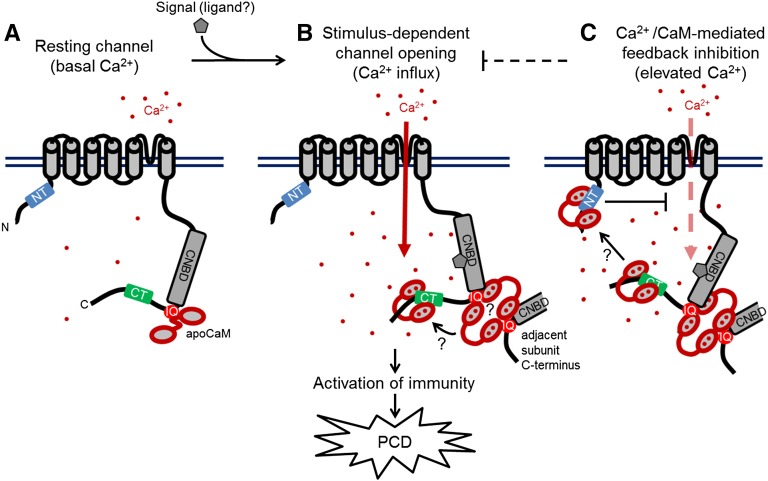
Figure 8.
Proposed Model of CNGC12 Regulation by CaM.
The model draws from the biophysical and physiological results of this study, previous data cited in the Discussion, and speculative components that currently remain unexplored, but which are compatible with our findings to date.
(A) At resting conditions and basal Ca2+ levels, the channel is calmodulated via interaction between CaM and the IQ motif.
(B) Upon perception of a signal (the molecular nature of which remains to be empirically determined), the channel opens and cytosolic Ca2+ levels increase. Given the necessity of CaM binding to the IQ motif for channel function as well as the higher-order interactions that occur between the IQ motif and Ca2+/CaM, channel activation may require CaM-dependent interactions between adjacent channel subunits as depicted. Furthermore, current data do not resolve whether individual CaM molecules move between different CaMBDs in a Ca2+-dependent manner or multiple CaMs can bind sites independently.
(C) We propose that at higher Ca2+ concentration, Ca2+/CaM provides inhibition of CNGC12 function via the NT CaMBD; whether this inhibition involves the bridging of channel termini remains unclear.